Features Brief
Veeva eTMF Feature: TMF Transfer
Feature: TMF Transfer

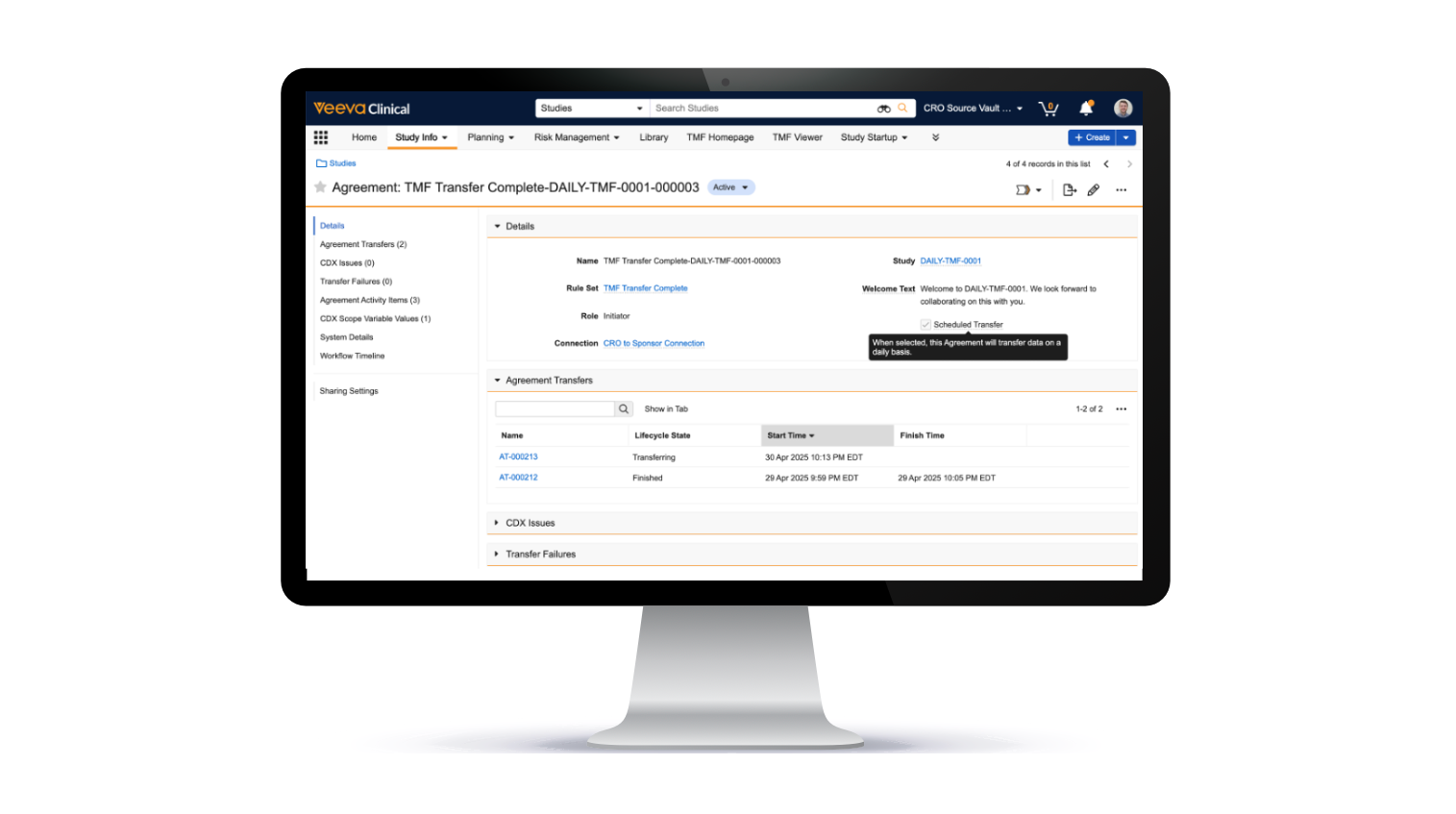
TMF Transfer enables study teams to quickly and accurately transfer TMF documents and related data. It eliminates the need for manual end-of-study migrations while improving sponsor oversight and fostering collaboration. The study country and study site records, approved TMF documents, and audit trail information seamlessly transfer between sponsor and CRO eTMF Vaults daily or at the end of the study.
Benefits
-
Streamline Data Transfers
Increase efficiency and eliminate the need for manual end-of-study migrations or external storage. Instead, digitally transfer between Vaults when needed. -
Increase TMF Quality
Migrate documents and data records for easy compliance without extensive mapping. Choose a daily transfer cadence to ensure oversight and quality throughout a study. -
Enhance Collaboration
Connect to other eTMF Vaults for additional transfers, whether from CRO to sponsor or sponsor to sponsor.
What Gets Transferred
After configuring the initial connection, transfer everything needed for a study to meet regulatory requirements without a costly manual migration. Control when the transfer happens, and what gets transferred. These documents and data records include:
- Study country and study site records
- Select country and site fields
- Steady state (approved) documents
- All approved versions of those documents
- Audit trails associated with those documents
- Document standard fields
- Document content and rendition data
Learn more about how Veeva eTMF improves efficiency and drives inspection readiness.
