Features Brief
Veeva LIMS for Virtual Biopharma Feature Brief
Modernized Quality Control with Veeva LIMS
Veeva LIMS is a comprehensive cloud application that unifies QC data management across biopharmas and their contract partners. It enables Quality Control to optimize stability study management, external QC results entry, trend analysis, CoA and Stability report generation, and publication of QC outcomes to Veeva Batch Release to accelerate the release of products.
Alongside other Quality Cloud applications, including QMS, QualityDocs, and Training, LIMS delivers the efficiency, GMP compliance, and scalability companies need for product development and commercial launch.
Benefits
-
Modernize QC Oversight
Streamline QC data management to break down silos across internal and external systems, teams, and data. -
Embrace Digital First
Replace paper processes and spreadsheets with a true-cloud application to increase efficiency and improve accuracy and inspection readiness. -
Improve Compliance
Reduce errors and improve speed with seamless quality processes and automated workflows.
Features
-
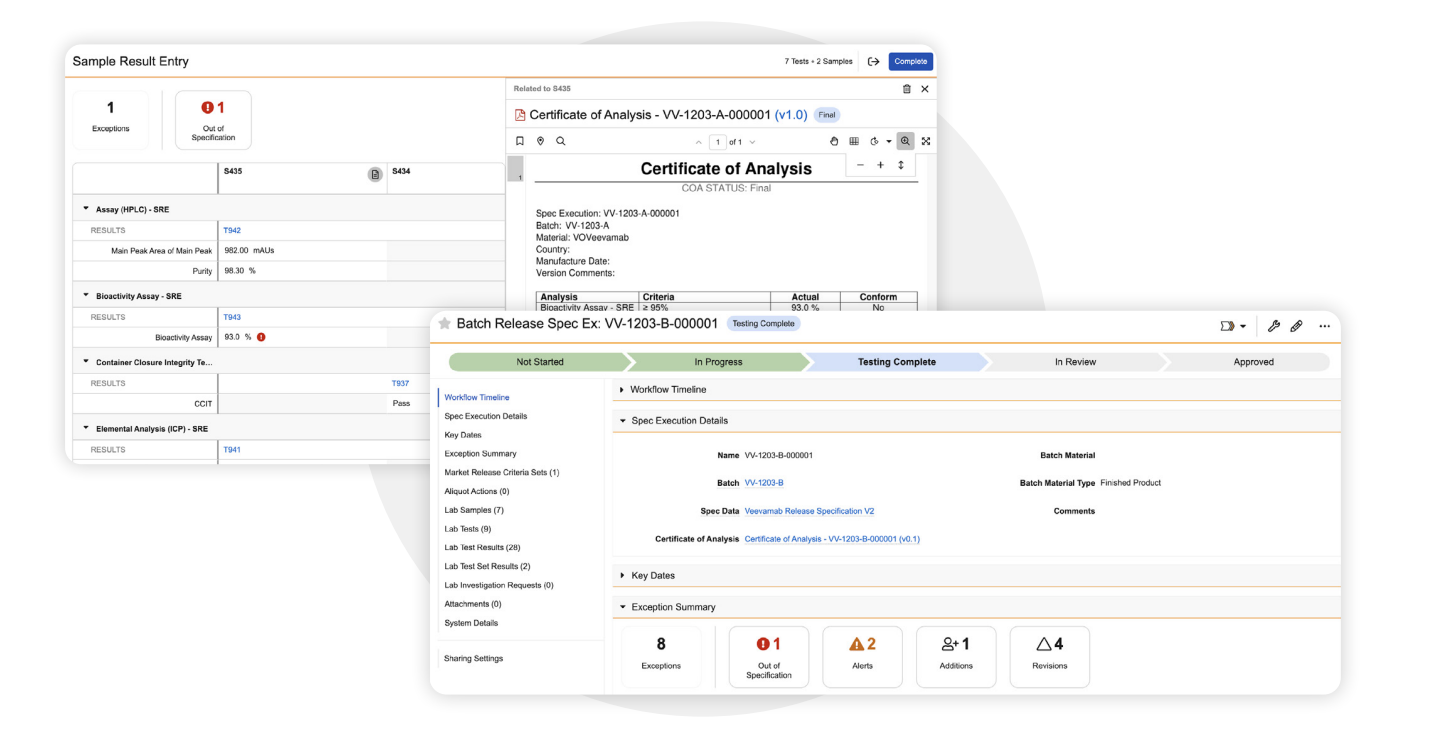
QC Batch Disposition
Manage the end-to-end QC batch disposition workflow, including digital sample results entry, trend analysis, review by exception, generation of the Certificate of Analysis (CoA) and publication of outcomes to Veeva Batch Release. -
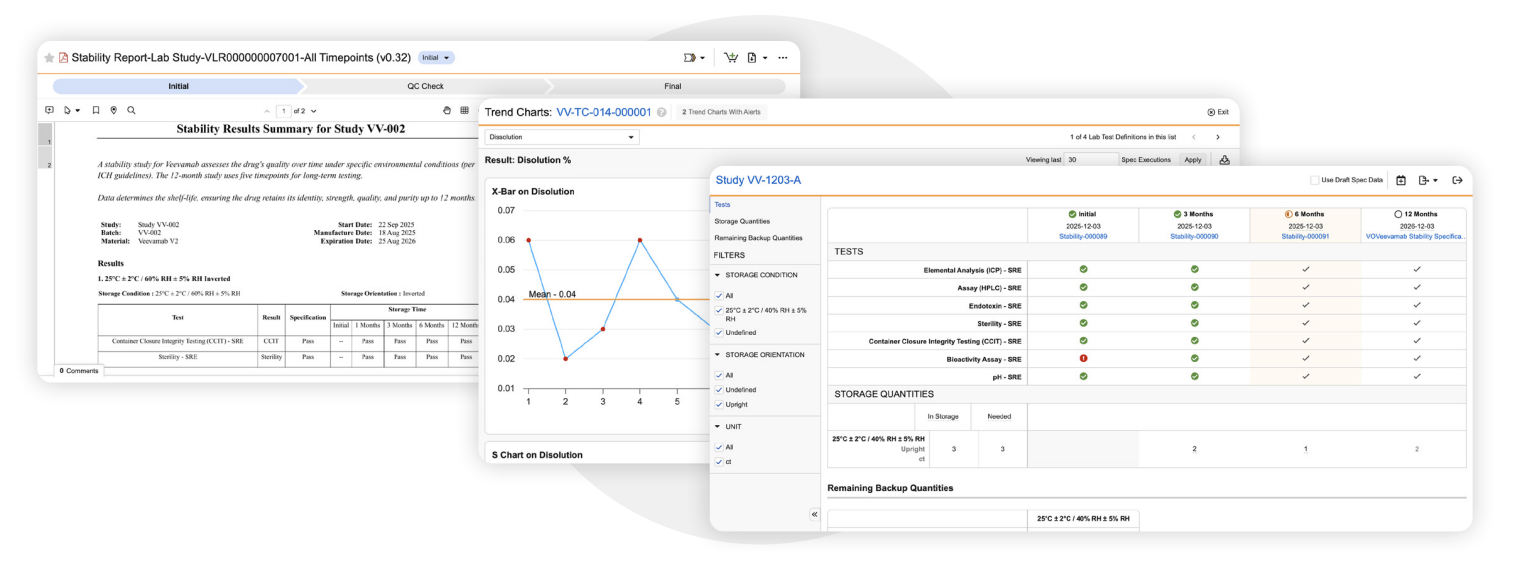
Trending
Quickly identify and respond to shifts in QC data with built-in trending capabilities. Automate the generation of trend and regression charts and detect out-of-control and out-of-trend results early, enabling proactive decision-making across outsourced manufacturing environments. -
Stability Study Management
Design, execute, and oversee stability studies with timepoint pull compliance, digital sample results entry, regression analysis, review by exception, and one-click generation of Stability Timepoint and Study Summary reports. -
Visual Design Data Builder
Simplify and improve the accuracy for the configuration of lab test definitions and specifications by guiding the design data administrator through stepwise workflow, visualizing the resulting configuration from an end-user’s perspective, and providing automated version difference comparison for streamlined design data review. -
Multi-Market Specification Evaluation
Easily evaluate test results against specification groups for multiple markets simultaneously to make release and product ship decisions and generate the corresponding Certificate of Analysis (COA).
About Veeva Quality Cloud
Veeva Quality Cloud accelerates the manufacturing of high-quality products to a greater number of patients. The cloud platform unifies applications, processes, and partners across content management, training, quality management systems (QMS), and QC lab solutions (LIMS).
