Veeva Study Startup
Accelerate Study Start-up, Enroll Patients Faster
Study Startup manages the start-up activities of a trial, including feasibility, qualification, and activation of research sites.
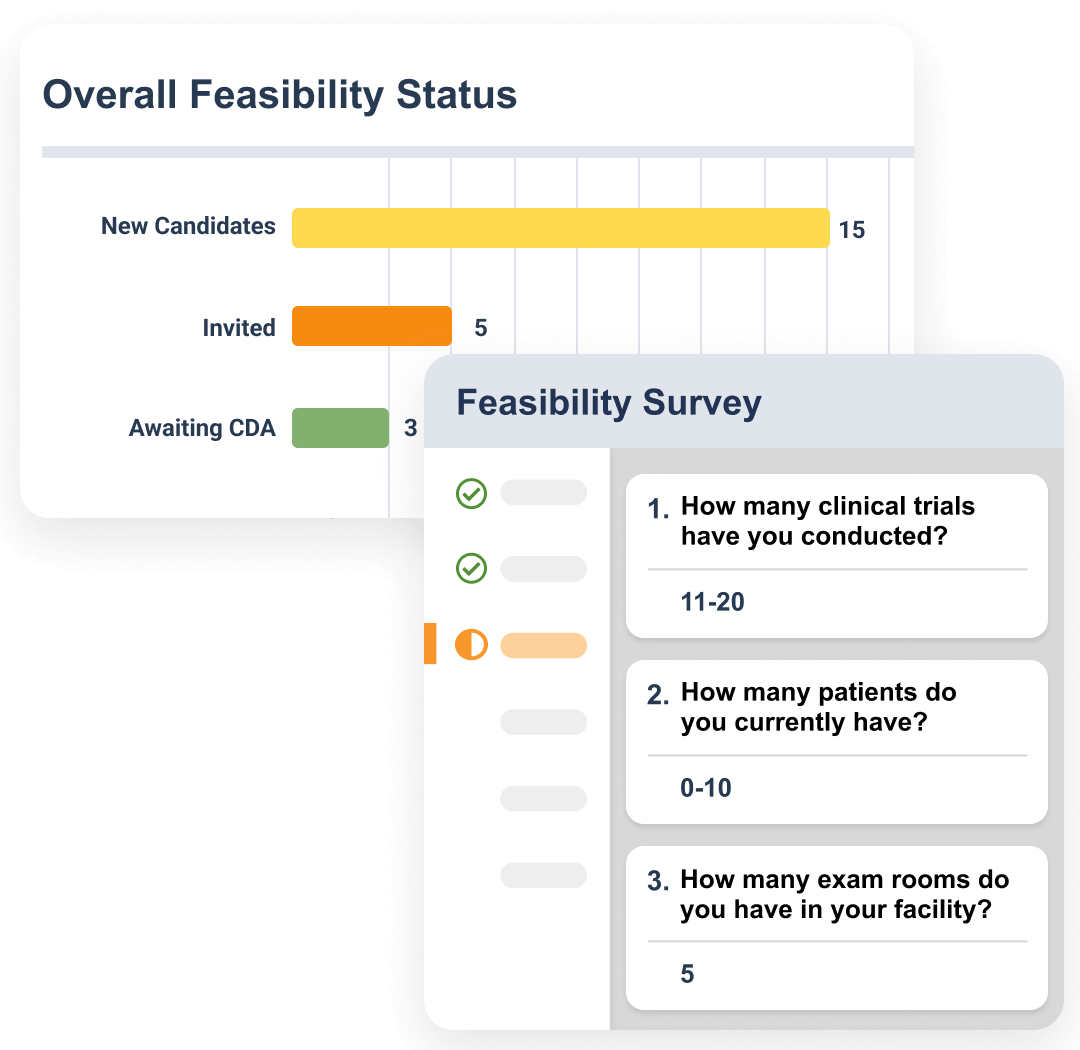
Feasibility surveys are sent to research sites during the site selection phase. Sites are automatically scored based on their response, and those selected are routed into the site activation process.
Study Startup drives site activation through standard tasks and milestones. These are controlled through provided and flexible country intelligence templates that specify the processes and documentation required before activating a site.
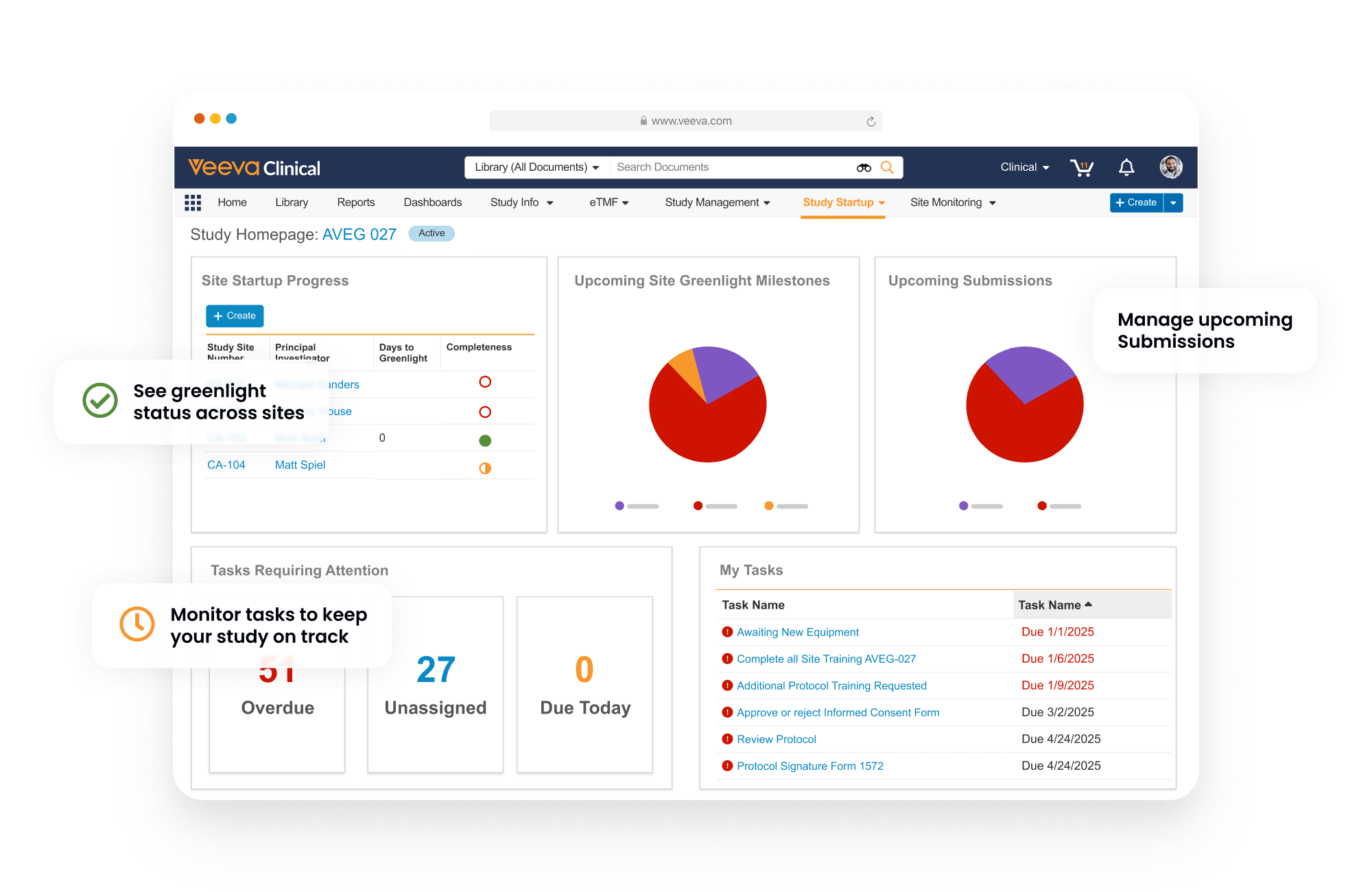
Information is collected, tracked, and presented in dashboard views to deliver visibility of start-up progress and timelines.
Announced 2015 Status Mature Customers 11-50
Learn how AstraZeneca saves 260 working days for monitors
Overview
Accelerate Study Start-up, Enroll Patients Faster
Study Startup manages the start-up activities of a trial, including feasibility, qualification, and activation of research sites.
Feasibility surveys are sent to research sites during the site selection phase. Sites are automatically scored based on their response, and those selected are routed into the site activation process.
Study Startup drives site activation through standard tasks and milestones. These are controlled through provided and flexible country intelligence templates that specify the processes and documentation required before activating a site.
Information is collected, tracked, and presented in dashboard views to deliver visibility of start-up progress and timelines.
Impact
Improve operational efficiency
50%
decrease in average days from site selected to ready for enrollment
45%
faster to enroll the first subject after site initiation
42%
decrease from protocol approved to first subject enrolled
Why Veeva Study Startup
Manage end-to-end processes in one system
Customer Success
Finding and activating the right sites faster










Resources
Explore and Learn
Read Features Brief
Bring Together Start-up Content and Data to Accelerate Time to Site Activation

Read Customer Story
Bristol Myers Squibb Accelerates Site Start-Up with Data-Driven Feasibility & Activation

Watch Demo
Study Startup Standard Questions
Read Case Study
Learn How AstraZeneca Optimizes with Data-driven Strategies

Watch Customer Video
Fortrea Eliminates Manual Study Start-up Processes

Watch Video
Know What’s on the Critical Path with Veeva Study Startup

Watch Video
Improve Performance by Measuring Study Start-up cycle Times

Read Article
Four Steps to Accelerate Study Start-up Cycles

