Veeva Introduces New Capabilities for Remote Drug Sampling in Veeva CRM Engage Meeting
Field reps can compliantly create sample orders and fulfill requests
from healthcare professionals during remote meetings
Rapid pace of Veeva innovation helps life sciences continue
supporting HCPs as in-person access remains limited around the world
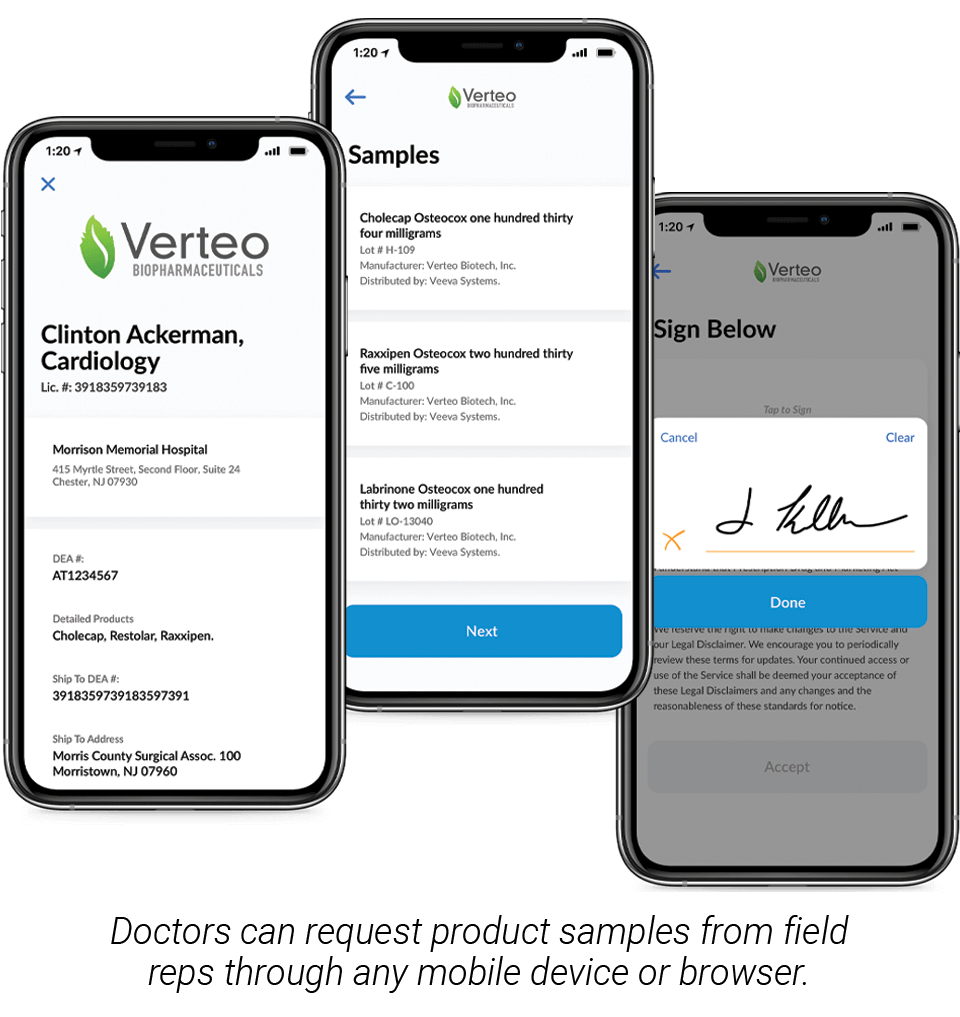
BARCELONA, Spain — 22 Apr, 2020 — Veeva Systems (NYSE: VEEV) today introduced new remote sampling capabilities in Veeva CRM Engage Meeting. Remote sampling enables field reps to capture and fulfill healthcare professionals’ (HCPs) sample requests, also known as business reply cards (BRCs), while hosting an online meeting. Now companies can complete drug sampling processes during remote meetings in compliance with 21 CFR Part 11 and PDMA requirements.
“Veeva CRM Engage Meeting is powering new ways of working remotely so field reps can help physicians maintain continuity in patient care,” said Arno Sosna, general manager of Veeva CRM. “Our dedication to product excellence and rapid innovation helps Veeva customers quickly deploy new digital capabilities and channels.”
Veeva CRM Engage Meeting makes online meetings with healthcare providers easy and compliant and, now with remote sampling, companies can deliver drug samples without the need for a face-to-face meeting. Reps can confirm license and address information, validate sample details, and save HCP signatures as part of the call record – the same compliant process they would follow during an in-person meeting. HCP signatures can be captured online across any mobile device or browser.
Remote medical inquiry management and consent management are also planned for Veeva CRM Engage Meeting. These new capabilities will allow field reps to compliantly capture and respond to HCP medical inquiries and enable HCPs to approve their communication preferences using Veeva CRM Engage Meeting.
These latest innovations will continue to help the industry in its rapid shift to digital. Since March 2020, Veeva has offered Veeva CRM Engage Meeting free to new customers so they can stay connected with HCPs. During March, life sciences companies connected with more than 100,000 HCPs online(1), up 2,347% compared to January. These remote meetings are also more in-depth, lasting an average of 13 minutes longer(2) than a typical face-to-face meeting.(3)
Remote sampling is available today in Veeva CRM Engage Meeting. Remote medical inquiry management and remote consent are expected to be available in the coming months. To learn more about Veeva CRM Engage Meeting, visit veeva.com/eu/engage-meeting.
Learn how Veeva is helping customers leverage digital to connect with HCPs at the upcoming Veeva Summit on 9 June, 2020. Pre-register for the virtual event and stay up-to-date on program details at veeva.com/Summit.
___________
(1) Based on internal data from Veeva HCP Pulse.
(2) Based on internal data from Veeva HCP Pulse.
(3) Pharma Sales Reps are Struggling – Here’s Why. BlueNovius. May 2018.
Additional Information
Connect with Veeva on LinkedIn: linkedin.com/company/veeva-systems
Follow @veeva_eu on Twitter: twitter.com/veeva_eu
Like Veeva on Facebook: facebook.com/veevasystems
About Veeva Systems
Veeva Systems Inc. is the leader in cloud-based software for the global life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 850 customers, ranging from the world's largest pharmaceutical companies to emerging biotechs. Veeva is headquartered in the San Francisco Bay Area, with offices throughout North America, Europe, Asia, and Latin America. For more information, visit veeva.com/eu.
Forward-looking Statements
This release contains forward-looking statements, including the market demand for and acceptance of Veeva’s products and services, the results from use of Veeva’s products and services, and general business conditions, particularly in the life sciences industry. Any forward-looking statements contained in this press release are based upon Veeva’s historical performance and its current plans, estimates, and expectations, and are not a representation that such plans, estimates, or expectations will be achieved. These forward-looking statements represent Veeva’s expectations as of the date of this press announcement. Subsequent events may cause these expectations to change, and Veeva disclaims any obligation to update the forward-looking statements in the future. These forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially. Additional risks and uncertainties that could affect Veeva’s financial results are included under the captions, “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in the company’s filing on Form 10-K for the period ended January 31, 2020. This is available on the company’s website at veeva.com under the Investors section and on the SEC’s website at sec.gov. Further information on potential risks that could affect actual results will be included in other filings Veeva makes with the SEC from time to time.
###
Contact:
Veeva Systems
pr@veeva.com