Blog
The Value of a Modern CTMS
Jan 07, 2020 | Henry Galio
Jan 07, 2020 | Henry Galio
In our previous blog, we discussed how you can assess your trial management operations process maturity. In this final blog, we’ll dive a bit deeper into the advancements in clinical trial technologies that have made it possible to address the challenges of legacy systems.
Managing today’s global trials requires an advanced, easy-to-use, and flexible CTMS that can deliver significant cost savings across monitoring, issue management, and oversight. See below for a few key benefits of modern cloud-based CTMS applications.
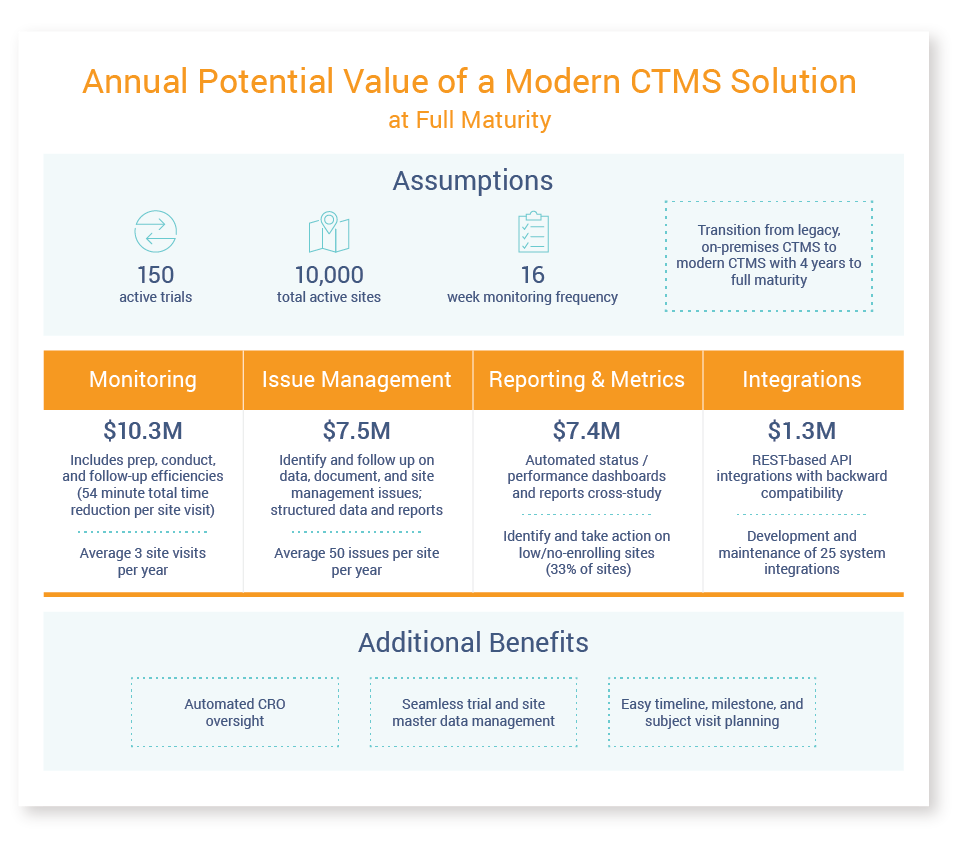
Achieve Monitoring Efficiencies
Modern clinical trial management systems on a unified platform eliminate silos, improve study visibility, and enable centralised monitoring and oversight needed to effectively manage and optimise trials. Role-based dashboards provide real-time insights, enabling users to take action at the point of decision without logging in and out of multiple systems. Configurable reports help identify and resolve issues immediately.
For example, role-based dashboards and a unified view across study activities make the monitoring process more efficient by better preparing CRAs to conduct study initiation visits and follow-up activities. It enables CRAs to prioritise critical tasks, track enrollment status for each site, and review open issues and missing documents. They can resolve issues and request missing documents without leaving the system.
Improve Issue Management and Get Greater Visibility
Effective trial management requires getting a complete, accurate, and global view of all operational data related to study planning, management, and reporting. With a real-time view across end-to-end clinical trial processes, a modern CTMS enables clinical teams to:
- Plan, track, and mitigate potential subject enrollment bottlenecks
- Identify and shut down low-enrolling and no-enrolling sites
- Proactively identify issues and take corrective action
- Achieve compliance through study and CRO oversight
Drive CTMS Innovations
A modern, cloud-based CTMS eliminates the need to purchase, deploy, and maintain on-premises IT assets and associated services, as cloud vendors take full responsibility for running applications and ensuring performance.
In addition to direct cost savings, shifting infrastructure responsibilities frees up your IT team to focus on more strategic initiatives. Other benefits of cloud-based solutions include:
- Rapid innovation – With a single version, customers of a true cloud application realise the benefits of best practices and new capabilities driven across the industry. True cloud solutions lead to better, highly tailored software with better quality and stability. The “innovation enabler” is the single software version running in the cloud.
- Stay current and compliant – The latest features and innovations are immediately available without spending time on system upgrades or custom IT development.
- Greater accessibility – Software can be accessed from anywhere, anytime, through any device.
- Faster deployment – Tailoring the application to your specific requirements is faster, as enterprise cloud applications are highly configurable by design.
Simplify and Streamline Clinical Operations
Modern, cloud-based CTMS applications unify clinical information and processes for greater efficiency and seamless exchange of trial information across CTMS, eTMF, and study start-up processes. Organisations can manage the entire end-to-end clinical trial process and gain a global view into trial activities, from study planning to closeout.
Watch our webinar to learn best practices for migrating to a modern CTMS application.
