Veeva Vault Submissions Publishing
Remove Bottlenecks with
Continuous Publishing
Dramatically speed up submission delivery.
为什么使用Vault Submissions Publishing
减少返工和延误
实现全面监督
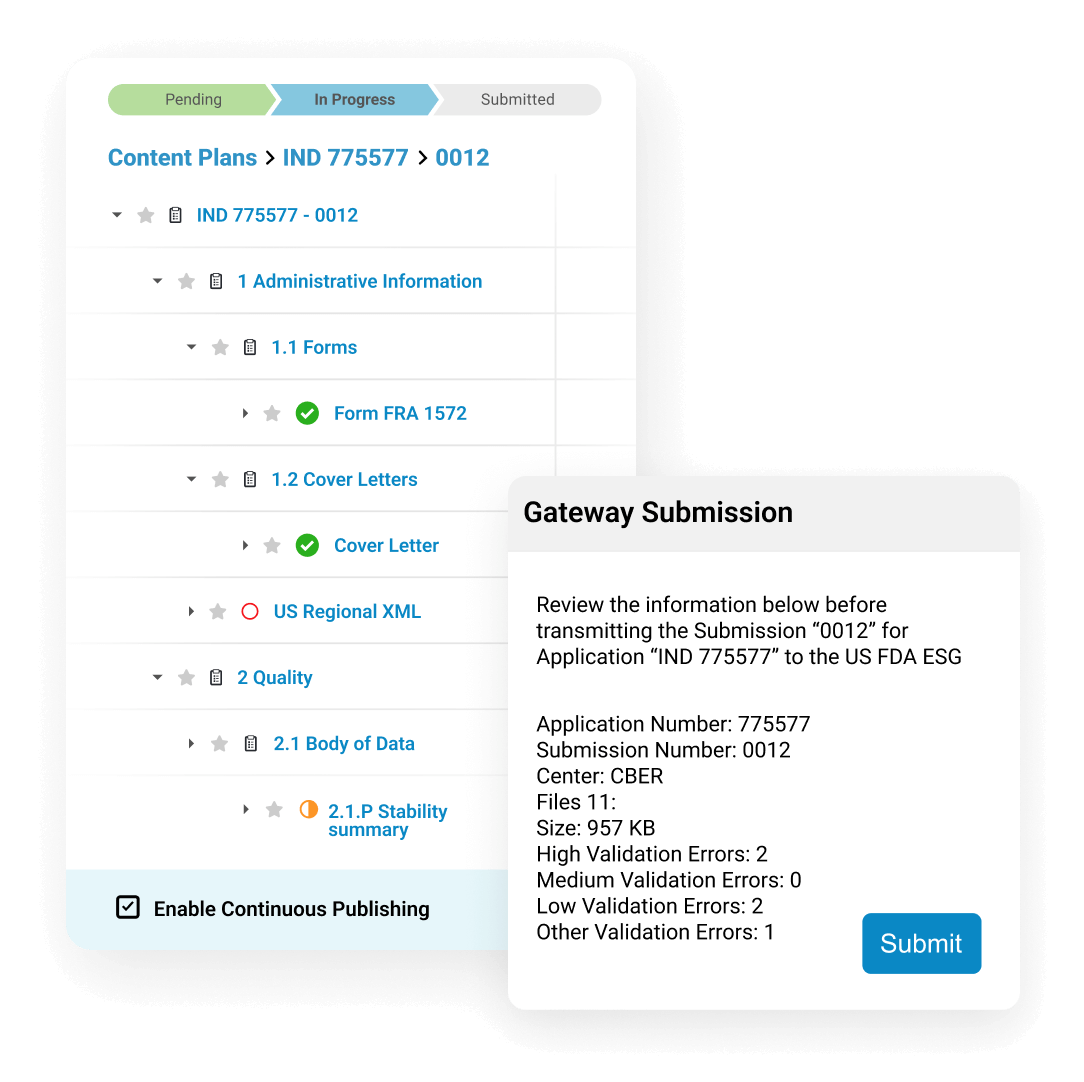
仅借助单个系统,即可管理端对端递交文件编制活动。减少错误和重复
始终使用正确的文档版本,无需手动跟踪或转移。提高效率
在递交文件的编写和审批过程中,在周期内即可尽早完成出版步骤。
省时省力
50%
递交文件创建时间缩减率
5周
NDA时间表缩减时长
700小时
直接网关递交文件时间缩减量