选择Veeva EDC
先进EDC提高敏捷性和效率
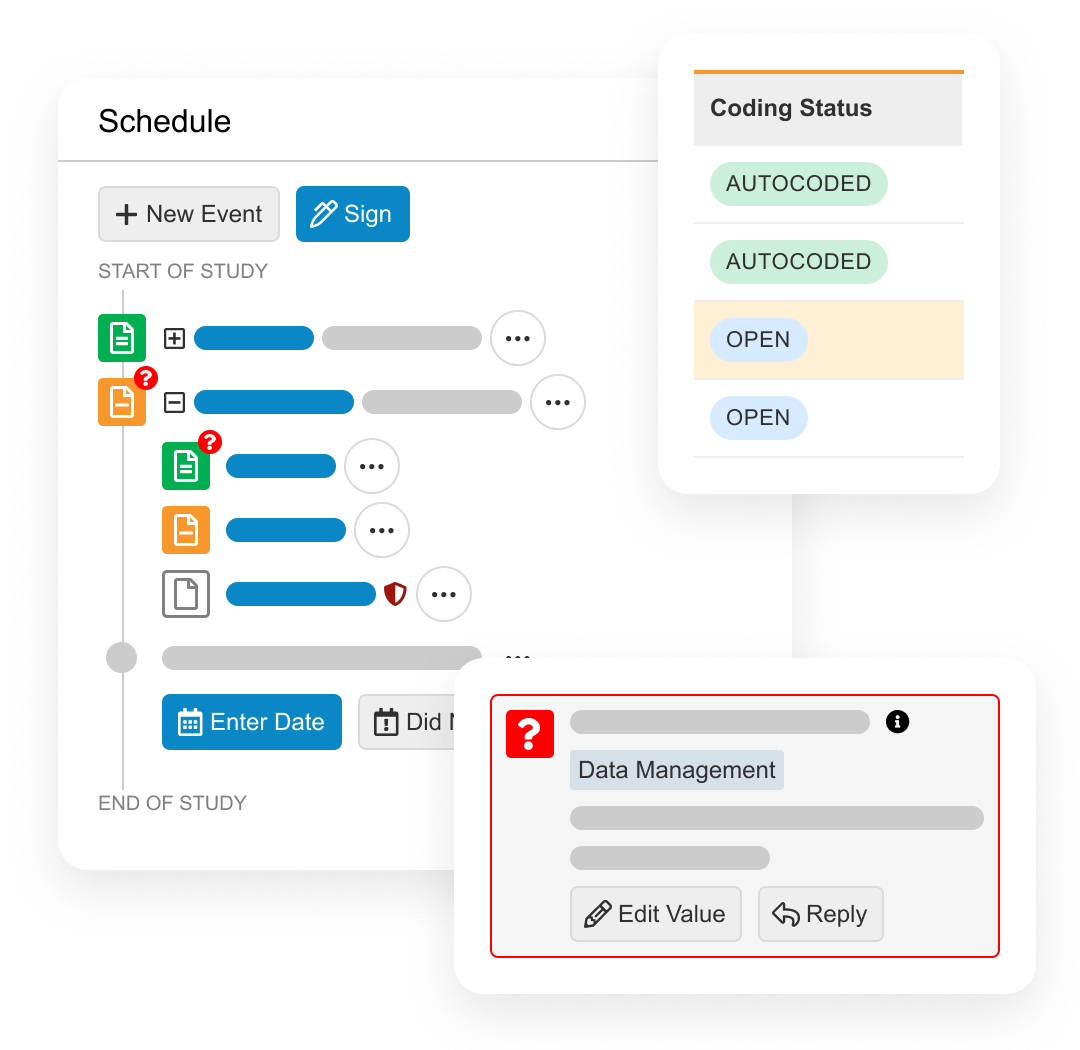
更快地构建研究
通过基于敏捷设计原则内置好的建库模块,设计复杂的研究。轻松获取更整洁的数据
通过基于角色的用户界面,促使用户采取行动,从而加快SDV和医学评估的速度。无需停机即可运行复杂的试验
对所有研究进行灵活设计和操作,包括主方案和适应性设计。连接临床团队
利用Vault Connections在整个研究团队中共享数据并进行协作。
经证明对复杂研究具有价值
50%
提升构建研究的速度
100%
无需自定义函数
9x
提升实施研究变更的速度
Interested in learning more about how Veeva can help?

联系我们