Veeva Validation Management
Fast and Unified Digital Validation Management
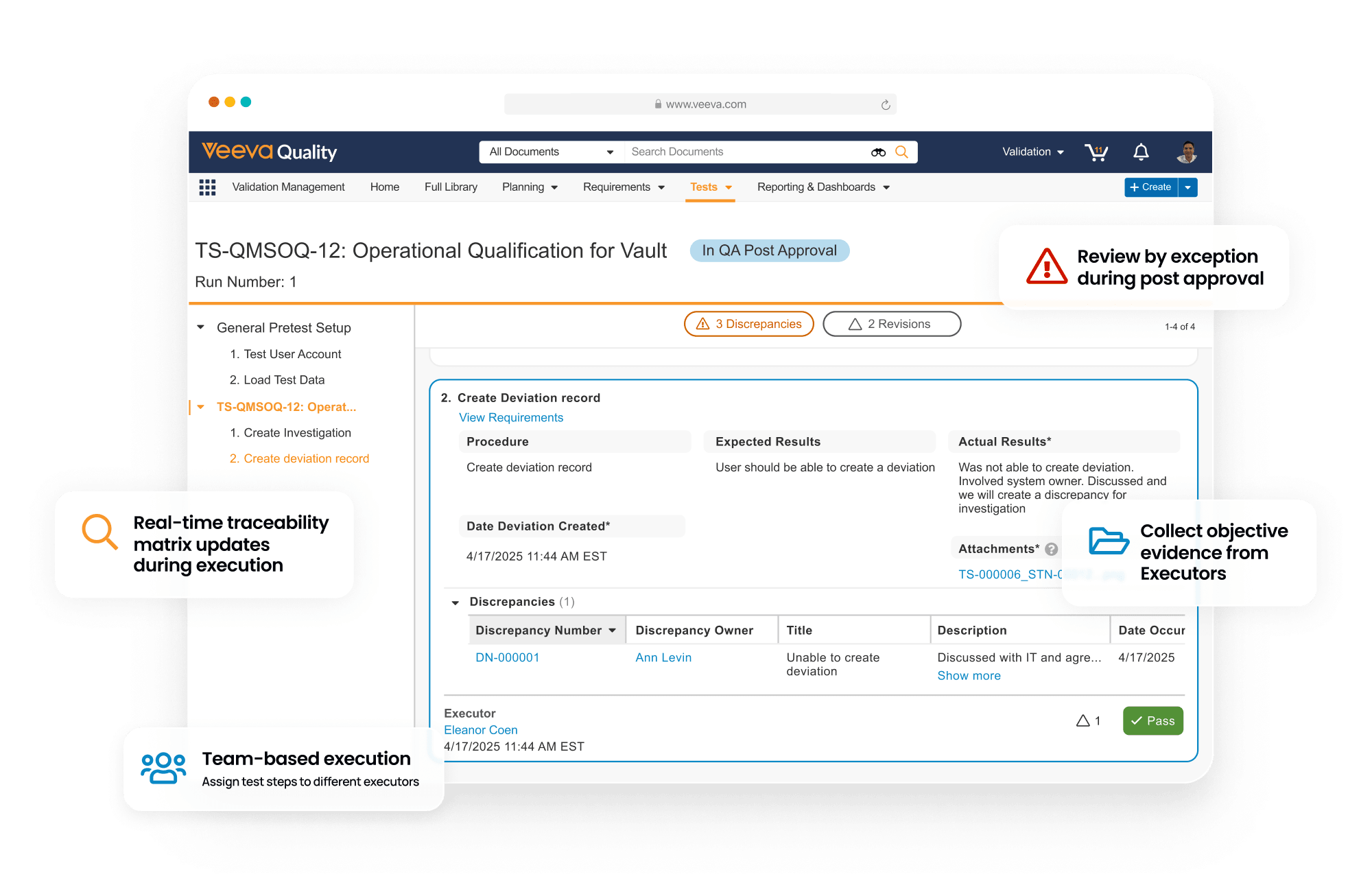
Validation Management is a digital solution for faster, accurate validation. It streamlines commissioning, qualification, and validation activities across computerized systems, facilities, utilities, equipment, and processes. It facilitates the tracking of system inventory, the management of requirements, and oversight of project deliverables. Validation activities can be easily created and approved, test scripts executed digitally, and traceability and summary reports generated automatically.
Validation Management is unified with QualityDocs and QMS to connect quality events and deliverables.
Announced 2021 Status Mature Customers 11-50
Overview
Fast and Unified Digital Validation Management
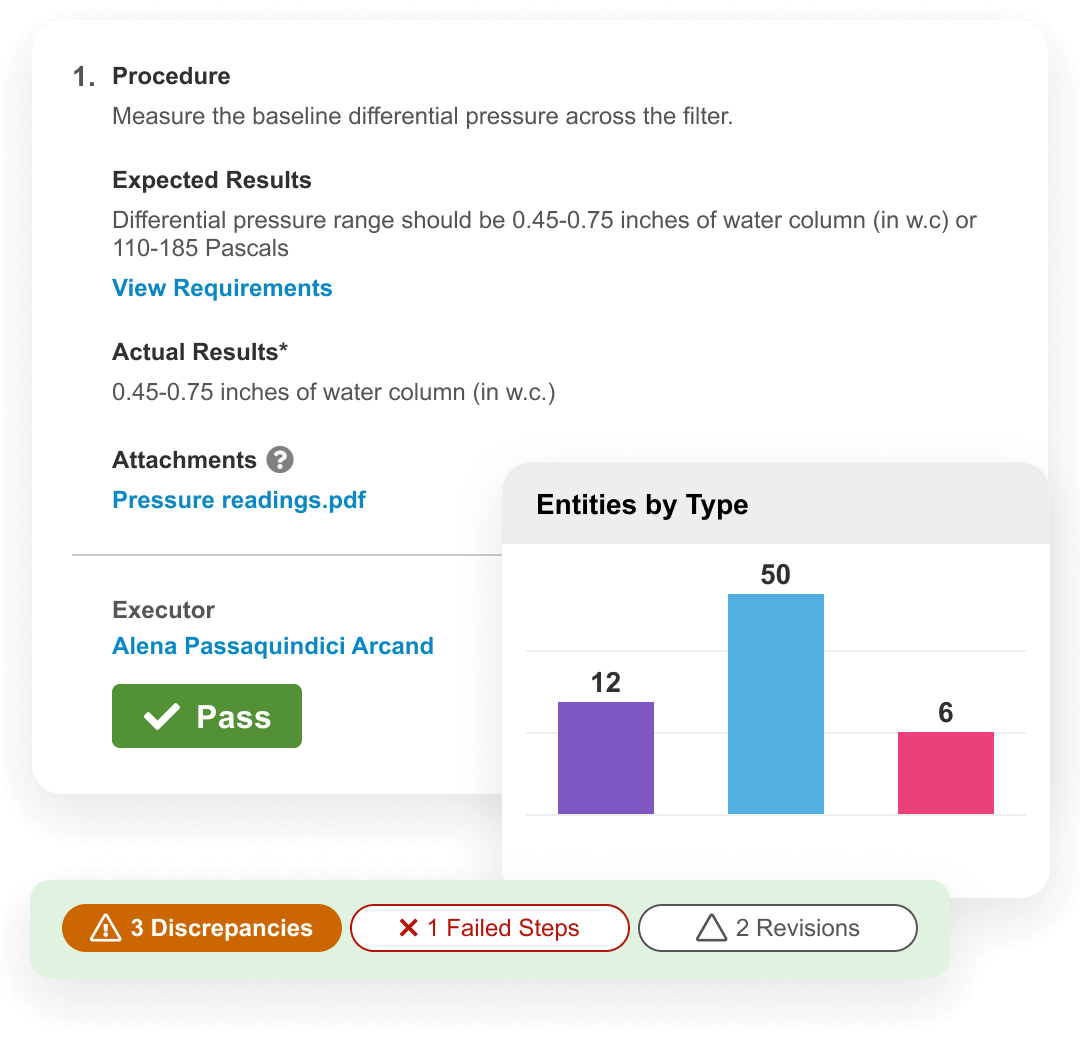
Validation Management is a digital solution for faster, accurate validation. It streamlines commissioning, qualification, and validation activities across computerized systems, facilities, utilities, equipment, and processes. It facilitates the tracking of system inventory, the management of requirements, and oversight of project deliverables. Validation activities can be easily created and approved, test scripts executed digitally, and traceability and summary reports generated automatically.
Validation Management is unified with QualityDocs and QMS to connect quality events and deliverables.
Why Veeva Validation Management
Faster Validation and Advanced Reporting
Resources
Explore and Learn
Read Features Brief
Veeva Veeva Validation Management Features Brief

Watch Video
Faster, More Accurate Digital Validation with Veeva Validation Management

Watch Video
AstraZeneca: Preparing for Rapid Manufacturing Growth With Unified Digital Validation

Read White Paper
A Business Case for Modernizing Validation Management

Read Blog
Beyond Digital: Taking a Data-Centric Approach to Validation Management

Read Blog
Expand Your Quality Ecosystem: Unifying Validation Workflows with QMS
