Blog
Driving Inspection Readiness for Outsourced Studies in Europe
Sep 06, 2022 | Pinar Bérénice Benét
Sep 06, 2022 | Pinar Bérénice Benét
The vast majority of European sponsors heavily or fully outsource clinical trial management to contract research organizations (CROs). This enables them to modernize and speed up clinical trials. However, irrespective of their outsourcing model sponsors have ultimate regulatory responsibility for the trial master file (TMF).
At the Veeva R&D and Quality Summit, Europe, two biopharmaceutical companies and a CRO shared how they successfully drive inspection readiness in different TMF operating models. In this article, we explore best practices by operating model for oversight and collaboration with study partners using Veeva Vault eTMF.
Model 1: Best practices to leverage CRO eTMF
One operating model involves CROs owning and managing the eTMF. KCR is a mid-sized CRO that encourages work in their own eTMF Vault. The reasons for this approach are that:- Vault eTMF is unified with Vault CTMS and connected with Vault EDC and other systems
- Study teams are trained on their internal systems and processes
- They have a monthly cadence for software and training updates
“Vault eTMF and Vault CTMS work very well together,” said Doug Bain, KCR’s chief technology officer. “We see great benefits — for example, our monitoring reports are automatically populated and delivered from the CTMS into the eTMF.”
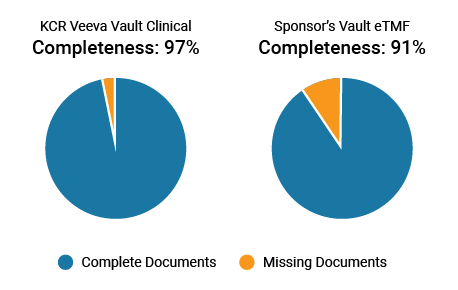
KCR enables sponsor oversight by providing sponsors access to their eTMF and maintaining granular reports and dashboards on metrics like completeness. Teams are more effective when working within their own processes and systems, recording a higher completeness metric of 97%, compared to 91% when working in sponsor systems. Sponsor-only documents can also be blinded in KCR’s system.
At the end of studies with sponsors that also have Vault eTMF, KCR can use the validated process through TMF Transfer to transfer TMF documents quickly and accurately and eliminate end-of-study migrations.
When leveraging CRO systems, sponsors should consider:- Where to store sponsor-owned documents, such as RFPs, internal SOPs, and oversight materials
- Which measures CROs provide for oversight, such as direct sponsor access and regular reports
- What migration strategy to use at the end of the study
Best practices for sponsor-owned eTMF
Other operating models involve sponsors owning an eTMF, either for sponsor documents or for the study as a whole.
Model 2: Sponsor and CRO eTMFs
Alvotech is a global biopharmaceutical company and maintains Vault eTMF for sponsor documents. Their CRO partners are responsible for migrating the TMF to them at the end of the study, where they can then archive it. Alvotech implemented this system to replace a paper filing system and improve inspection readiness, as well as enable CRO partners to continue working into their own systems.
“A CRO generally has its own systems and SOPs geared toward their processes,” said Ruth Ruffieux, Alvotech’s head of clinical operations. “As a small biotech, it’s difficult to ask a big or mid-size CRO to stop doing what they’re doing and work in our system — and that was one of the main reasons why we selected this model. It’s a good starting point for growing biotechs.”
Model 3: Sponsor uses in-house eTMF
argenx, a global biopharmaceutical company, is evaluating its long-term strategy to have CROs work into its sponsor-owned Vault eTMF. As the clinical trial volume and number of CRO partners have increased (they are currently running 30 trials), they are now evaluating this model to ensure a single source of truth.
“In recent inspections, it could take us up to four hours to get a document because we would need to identify where it was stored and whether we had direct access,” said Melissa De Swaef, argenx’s head of clinical operations processes and systems. “We hope to eliminate these steps with a single source of truth and CRO access, as well as streamline operations with the TMF Index functionality to simplify navigation of our eTMF.”
If the company rolls out this model to all trials, it predicts a five-year 100% return on investment due to eliminating end-of-study migrations, reducing audit and inspection preparation time, and managing documents more effectively across parties.
- How to secure CRO buy-in to work in their system, as well as train and provide access management for those CROs
- How to ensure active CRO management in their sponsor-owned eTMF
- What migration strategy to use if they transfer CRO documents into their own eTMF
- How to balance visibility and oversight with CRO efficiency
Sponsors can consider a variety of factors to decide which model best suits their organization, either as a whole or on a study-by-study basis. These factors include projected clinical trial volume and complexity, the number of CROs and other study partners, and CRO competencies.
For more best practices and eTMF trends, access on-demand sessions from the inaugural TMF Innovation Forum and contact your Veeva Account Partner.
About Pinar Benét
Pinar Benét is a senior director of strategy for clinical operations at Veeva Systems. She has more than 15 years of experience in life sciences, working with pharma sponsors and CROs across multiple therapeutic areas in Europe. Pinar is actively involved in making sure Veeva solutions and services enable customer success.