Blog
Overcoming RBQM Adoption Challenges
Aug 17, 2021 | Lauren Garson
Aug 17, 2021 | Lauren Garson
In our previous blog, we discussed adopting a risk-based approach to monitoring to prepare CRAs for the hybrid trials of the future. This blog will explore common adoption challenges and share tips to garner internal support for risk-based initiatives.
Risk-based quality management (RBQM) isn’t a new concept. Section 5.0 of the ICH GCP E6 (R2) Addendum introduced in November 2016 recommends that sponsors implement a quality system to ensure subject safety and reliability of trial results, and suggests a risk-based approach with these components:
- Critical Process and Data Identification
- Risk Identification
- Risk Evaluation
- Risk Control
- Risk Communication
- Risk Review
- Risk Reporting
Yet data from the Association of Clinical Research Organizations (ACRO) shows that industry adoption is less widespread than expected, with few studies incorporating all components of RBQM and many companies taking a fragmented approach to implementation and execution.1
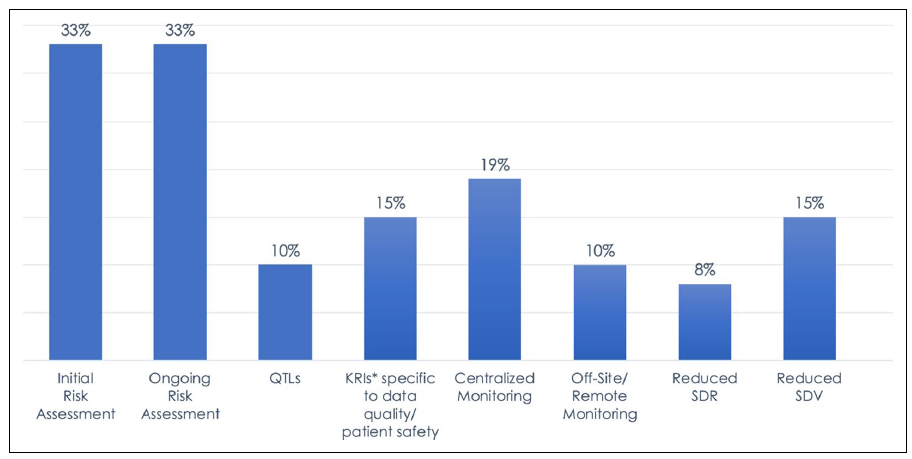
Figure 1: 2019 Landscape of RBM/RBQM Components in Clinical Trials. Data represents the percentage of all 6,513 trials included in the survey.
The challenge is there hasn’t been clear guidance from the FDA and other regulatory bodies on what exactly sponsors should do to adopt a risk-based approach that also satisfies inspection requirements, which has caused many companies to struggle with implementation and adoption. Other barriers to widespread RBQM adoption include:
- Lack of budget to invest in RBQM technology
- Resources lack the skills and knowledge to be successful with RBQM
- Organizational reluctance to change and adopt a new monitoring approach
- Trial complexity impacts workflows and cross-functional alignment, hindering RBQM programs
- Failed RBQM initiatives in the past
Companies need to take a holistic view and implement all aspects of RBQM to proactively mitigate risks and realize the full potential to enhance data quality. Here are some tips to get your organization on the path to successful RBQM adoption:
- Secure support from executive leadership to drive organizational change. Building a business case to justify the cost and effort helps decision-makers see the value of the initiative and understand how it can positively impact trial quality, patient safety, and study efficiency.
- Be the evangelist for the right processes and technology in your organization. Identify optimization areas to ensure technology isn’t supporting a broken process. Evaluate processes from a holistic lens that isn’t constrained by your organization’s functional structure.
- Gain cross-functional buy-in and educate stakeholders on roles and responsibilities. Success with RBQM extends beyond CRAs and clinical operations leaders, requiring coordination and collaboration with others such as clinical data management, pharmacovigilance, and statistics. These stakeholders must understand expectations so their expertise can be leveraged in the development of risk assessment templates, key risk indicators (KRIs), quality tolerance limits (QTLs), monitoring plans, reporting, and other components within a comprehensive RBQM program.
- Establish baseline metrics to measure program progress and success. Organizations should review progress in key areas such as data quality and efficiency to assess effectiveness. For example, tracking the number of protocol deviations and audit findings when there is a reduction in source data review (SDR) and source data verification (SDV) can help organizations determine if moving to centralized and remote monitoring has a material impact on quality and patient safety.
COVID has taught the industry we can rapidly adjust to change and be flexible without sacrificing trial quality and patient safety. It is possible to shift to RBQM without impacting monitoring effectiveness. To learn more about risk-based monitoring and quality management in clinical trials, read this report I co-authored with my industry peers from ACRO.
My final blog in this series, which will focus on risk-based study management in Veeva Vault CTMS, will be published soon — stay tuned!
___________________________________
1. Risk-Based Monitoring in Clinical Trials: Past, Present, and Future. ACRO. April 2021.
