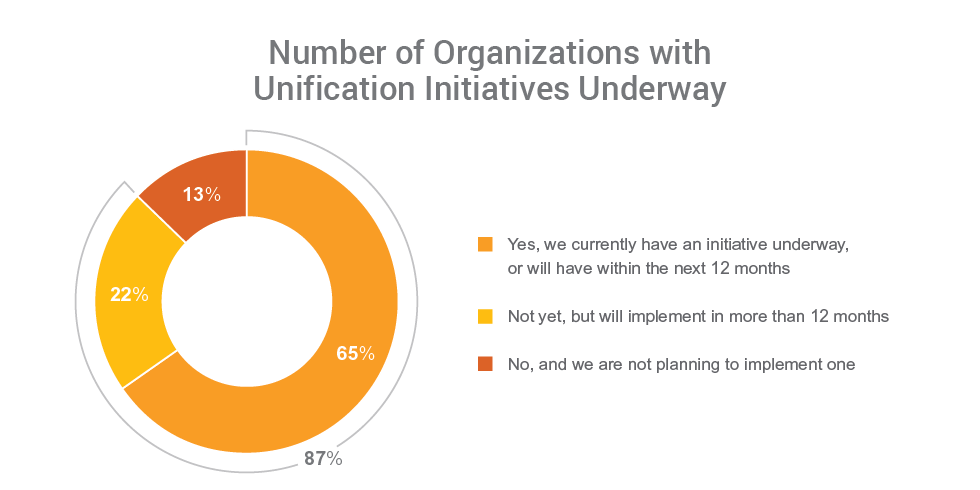
Findings show the industry is taking action to unify their clinical trial systems and drive end-to-end processes for better visibility and improved study execution. Nearly all (99%) respondents report the need to unify clinical applications, and 87% say their organizations have, or plan to have, an initiative in place to do so.
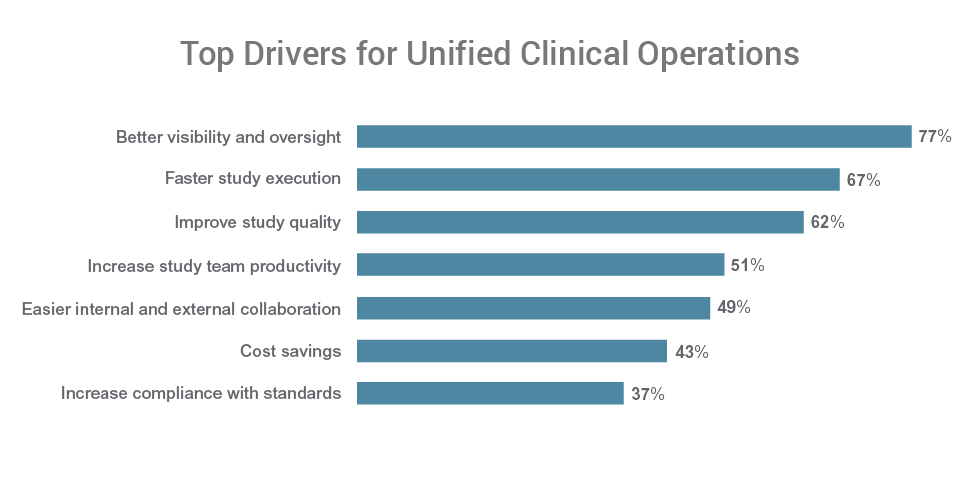
Better visibility is a top driver for unifying clinical applications for 77% of respondents. Over half of respondents cite faster study execution (67%), improved study quality (62%), and increased productivity (51%) among the primary reasons to unify their clinical applications.
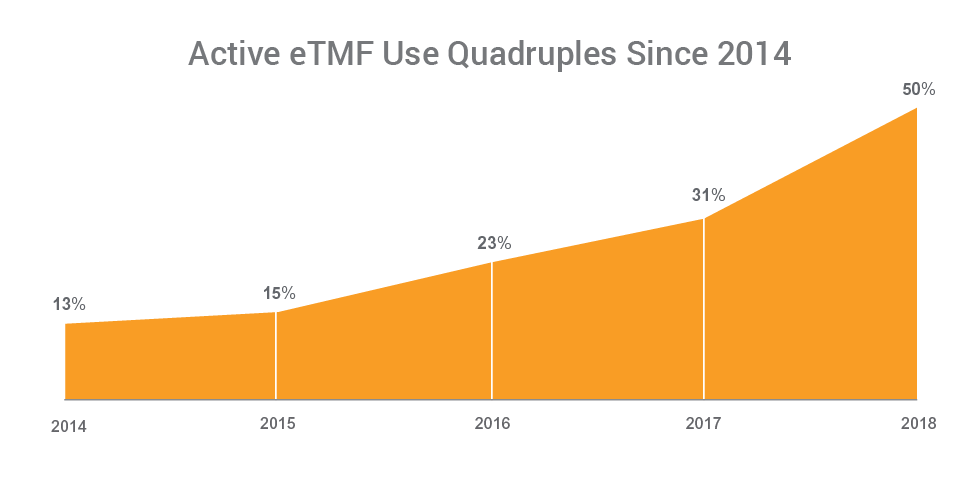
The number of organizations now using an eTMF application has quadrupled since 2014. Half of sponsors (50%) now use a purpose-built eTMF application versus 13% in 2014, and 31% in 2017. The increase in the use of eTMF applications is matched by a sharp decline in the use of content management systems, signaling a shift away from general-purpose methods used in ‘passive’ models, towards ‘active’ TMF management.
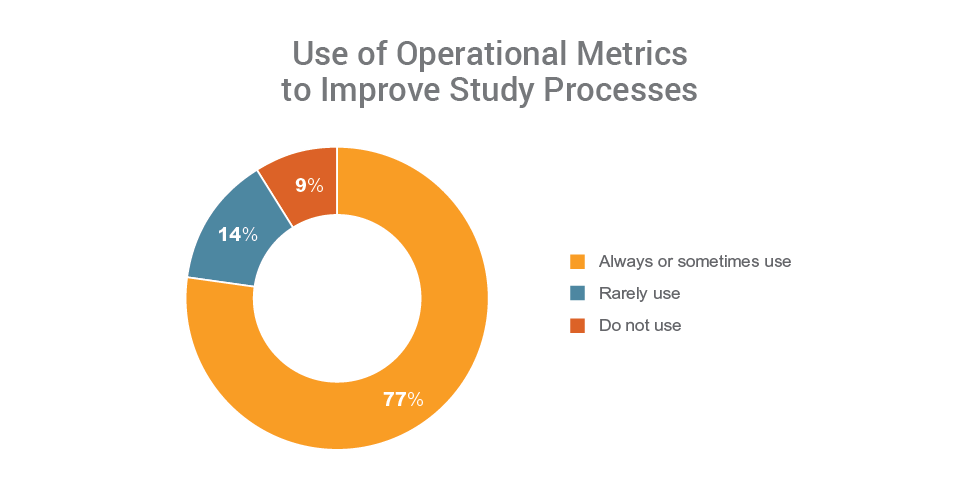
Organizations that use metrics (77%) report fewer challenges with clinical operations and are four times more likely to have programs in place to unify their clinical applications than those not using metrics. Those that have programs in place to unify their clinical landscape are also more likely to use operational metrics to measure performance, manage risk, and implement process improvements.
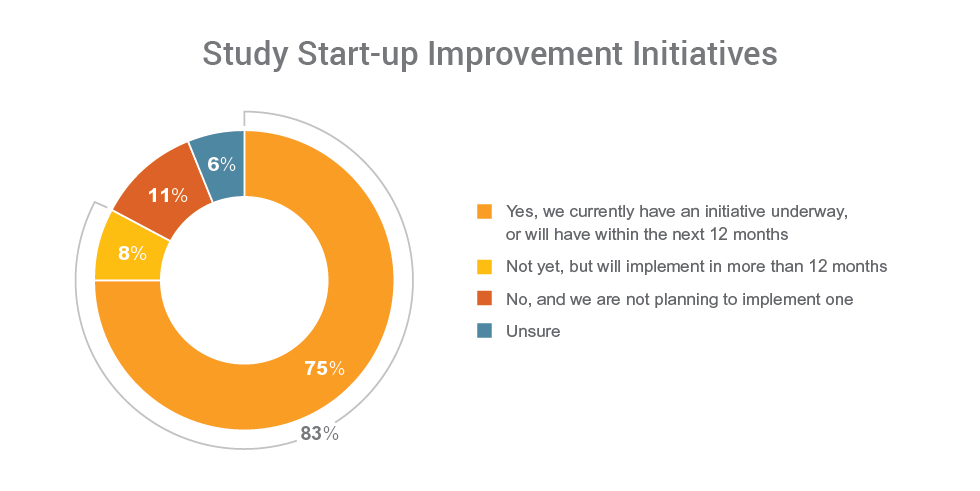
Consistent with the aim on improving study execution, study start-up is a priority focus. The data shows that 83% of organizations have an initiative underway, or will within the next year or more, to improve study start-up processes.
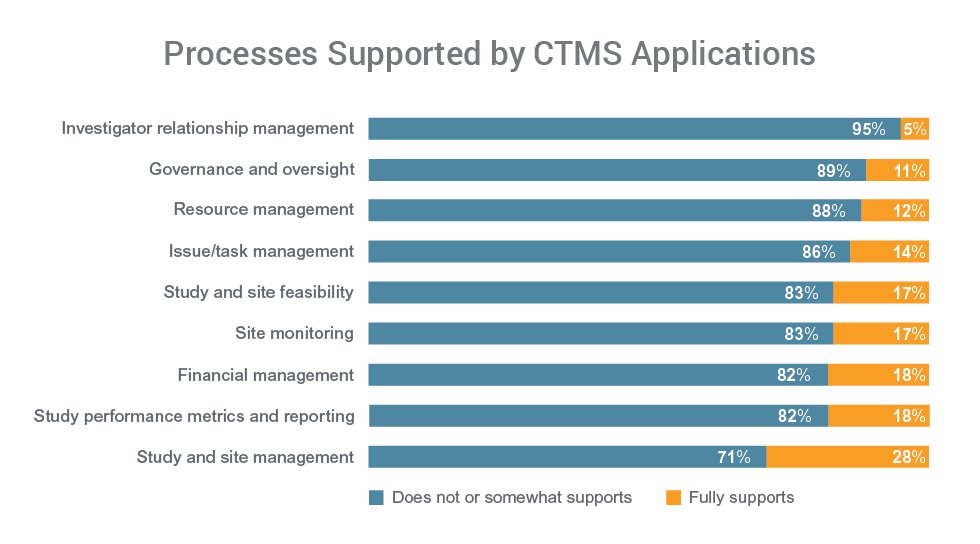
All respondents say they want to improve the use of CTMS in study operations. CTMS system issues are a limiting factor for 84% of respondents. The vast majority have CTMS applications that can’t fully support a range of key functions including governance and oversight (89%), resource management (88%), and issue and task management (86%).