Blog
Top Safety Topics at Upcoming Veeva R&D and Quality Summit
May 23, 2022 | Sharmila Sabaratnam
May 23, 2022 | Sharmila Sabaratnam
What is driving current and future changes to the pharmacovigilance operating model?
Scaling pharmacovigilance (PV) operations is a top concern for many safety teams and is a primary theme at Veeva’s in-person European R&D and Quality Summit. This year’s safety sessions will address key questions, such as how to:- Reimagine safety organizations to support growing case volumes and benefit-risk requirements
- Continue to meet regulatory compliance and manage risk while enabling PV transformation globally
- Leverage cloud technology to enhance data access and quality, speed, and reduce hidden costs of outsourcing to improve value
Transforming pharmacovigilance at LEO Pharma
Safety is complex with global operating models and a multi-stakeholder environment, demanding strong governance and more efficient processes.1 Companies are reevaluating their approach to pharmacovigilance to better scale and improve patient outcomes. Hear LEO Pharma’s safety vision, technology’s changing role, and how they are developing a foundation for transformation.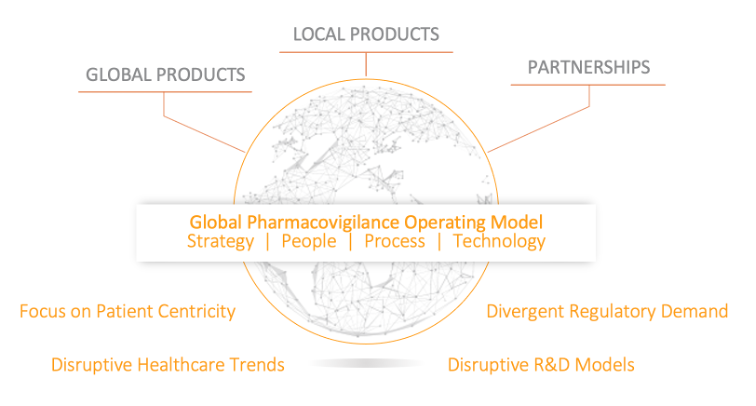
Getting ahead of regulatory and inspection trends
With increasing data requirements, localized regulations, and inspections of cross-functional safety processes and IT system landscape, it is even more difficult for pharma companies to remain inspection ready. Industry experts will discuss top safety concerns, EU QPPVs, and corporate compliance officers and explore how modern technology is enabling new approaches to a global safety database, safety data exchange, and content management to improve oversight and compliance. Armed with this information, pharmacovigilance organizations can better navigate the complex regulatory environment.Building PV outsourcing operations for scalability and quality
Pharma companies are outsourcing more PV activities to address a growing number of adverse events, data sources, and regulatory requirements. Services providers and CROs are leveraging technology to meet growing customer demand while reducing costs and providing a higher level of service. A panel of CROs–Arriello, Biomapas, and TFS—will share their PV strategies and how they are using Veeva Vault Safety to enable automation, data transparency, operational oversight, and help build client trust for successful working relationships.Veeva Vault Safety Suite product roadmap
Case processing comprises 40% to 85% of PV budgets and there is significant opportunity to drive greater productivity by streamlining or eliminating manual work.2 Get a first look at how Veeva approaches low-touch and touchless case processing for EMA and MHRA and reduces the burden of staying current with new regulations such as the European Directorate for the Quality of Medicines and Healthcare (EDQM) terminologies for dose forms and routes of administration.Join safety professionals at the Veeva R&D and Quality Summit in Zurich on June 8, 2022, to learn more about modernizing pharmacovigilance for seamless scalability.
___________________________________
[1] “Reimagine Patient Safety 2030. Oversight and Medical Governance Through a Unified Safety Platform.” DIA Global Forum for Qualified Persons for Pharmacovigilance (QPPV).
[2] “Transforming Pharmacovigilance Systems,” Deloitte US/Lifesciences and Healthcare White Paper, 2018
