Veeva Clinical Platform
Simplify and
Standardize Clinical Trials
Better efficiency and experience for sponsors, sites, and patients
Announcing Veeva eSource to eliminate paper and streamline data flow
Veeva Clinical Platform
-
Efficient for Sponsors
Run studies faster with a complete,
high quality platform -
Simpler for Sites
Reduce effort and connect with sponsors
on a standard platform -
Better for Patients
Keep patients connected through
one application
Products
Connected Products in a
Complete Clinical Platform
Clinical Operations
Unify clinical systems and processes on a single cloud platform to enable end-to-end trial management.
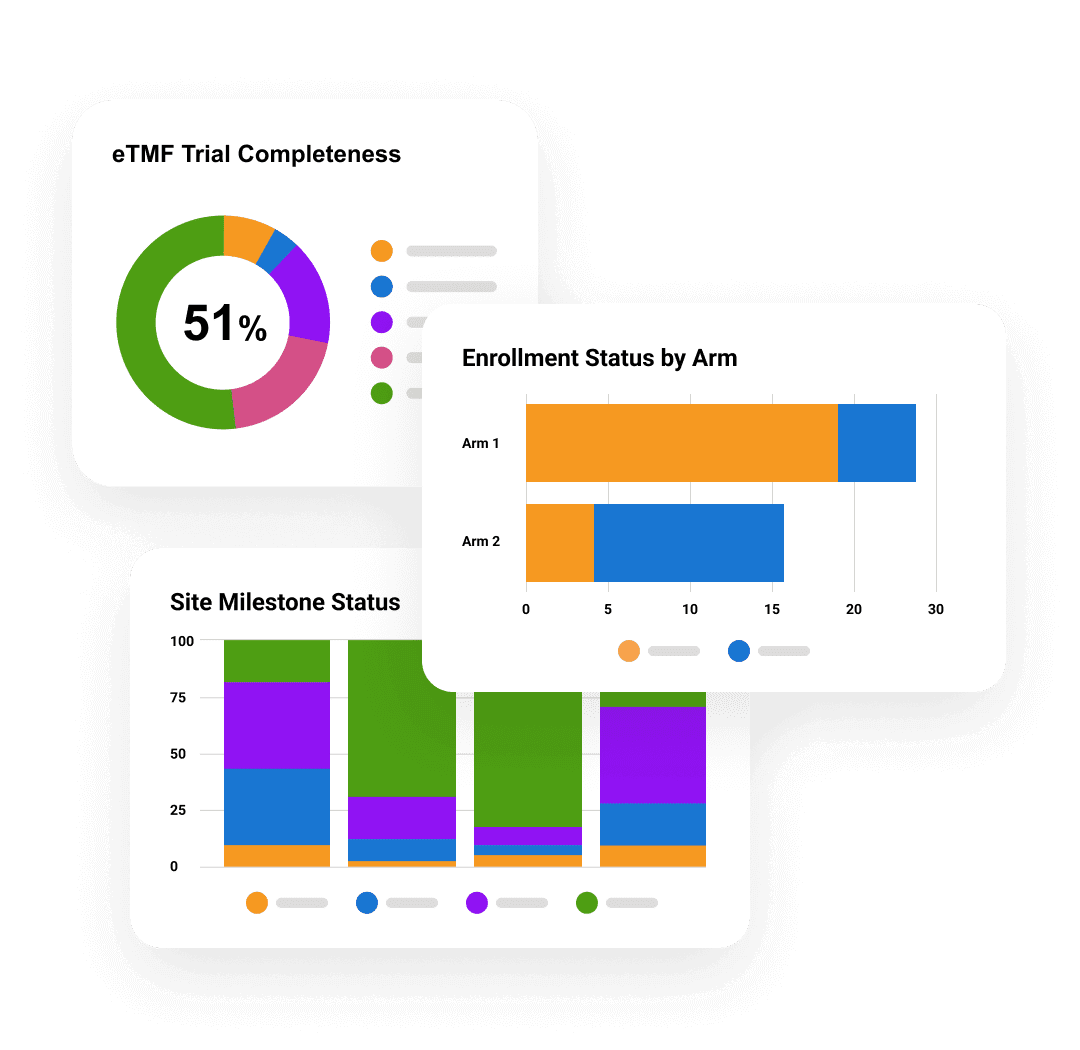
Veeva eTMF
Ensure TMF quality, timeliness, and completeness.
Veeva CTMS
Optimize trial management and monitoring for all studies.
Veeva Payments
Streamline payments to clinical research sites.
Veeva Study Startup
Simplify site selection and speed study start-up.
Veeva Site Connect
Automate document exchange during start-up, execution, and closeout.
Veeva Study Training
Manage GCP and study-specific training.
Veeva Disclosures
Share registrations and results disclosures with registries.
Veeva OpenData Clinical
Global reference data of sites, investigators, and their affiliations.
Clinical Data
Accelerate study timelines with modern applications.
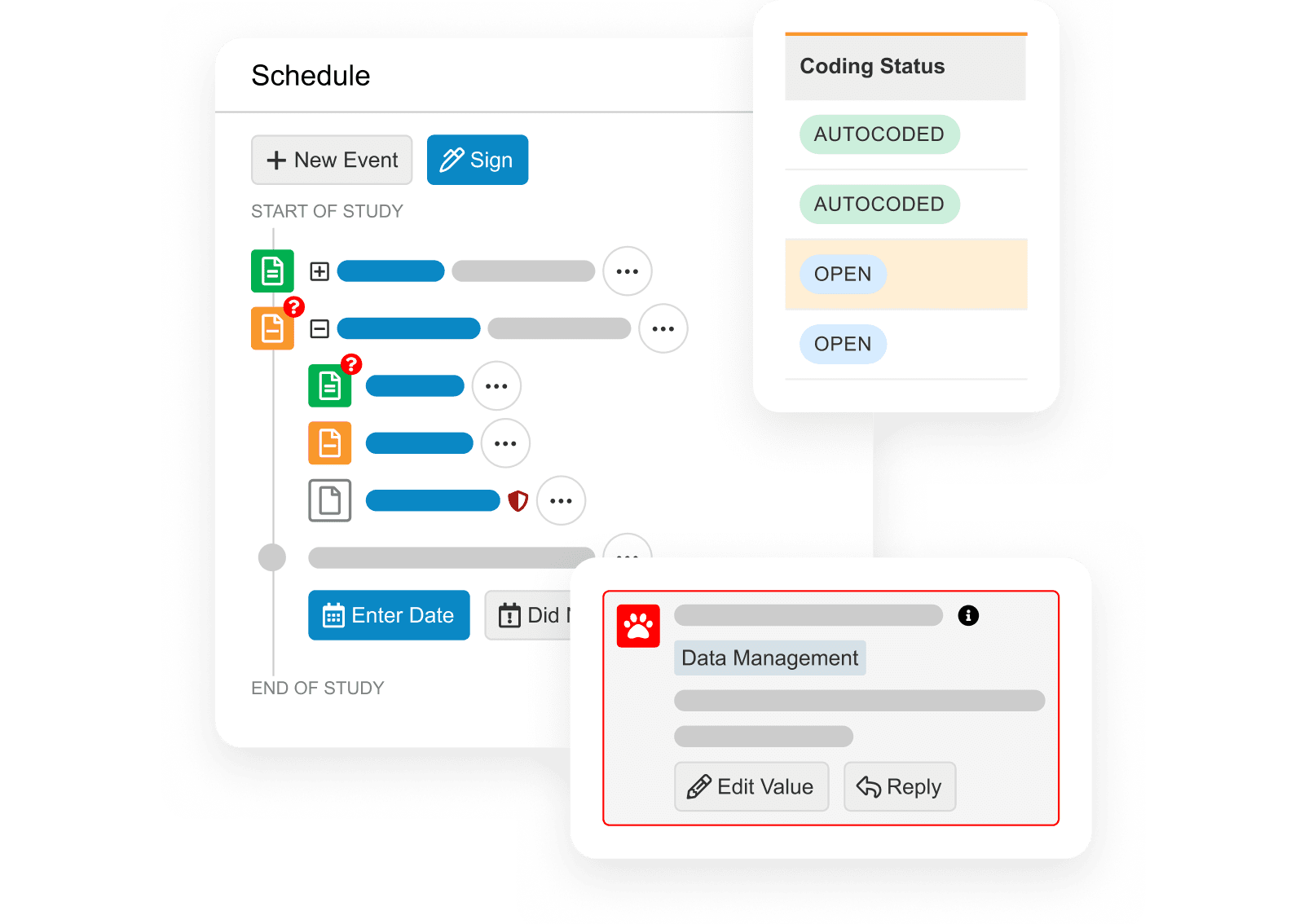
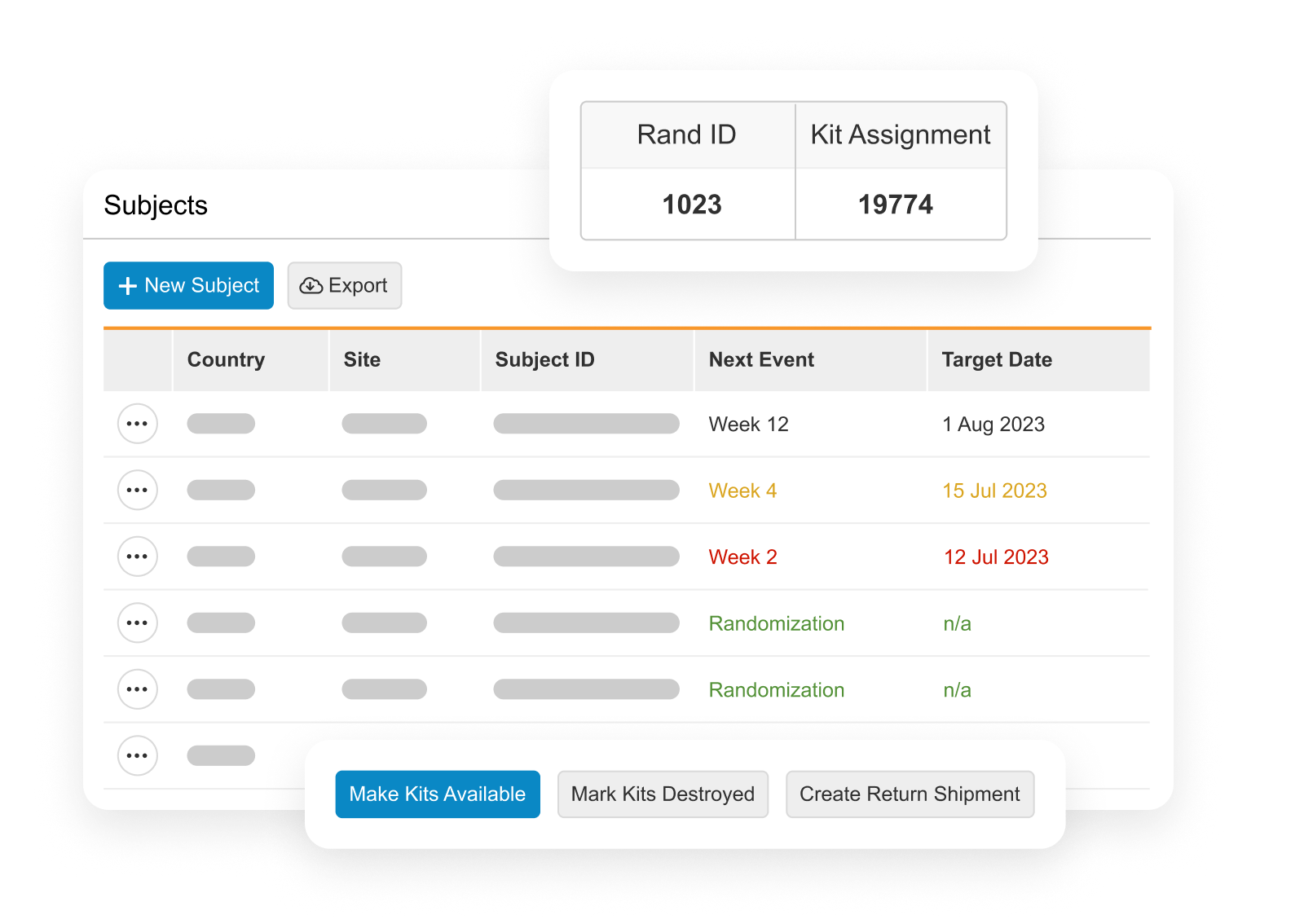
Veeva RTSM
Enterprise standard solution for all trial types.
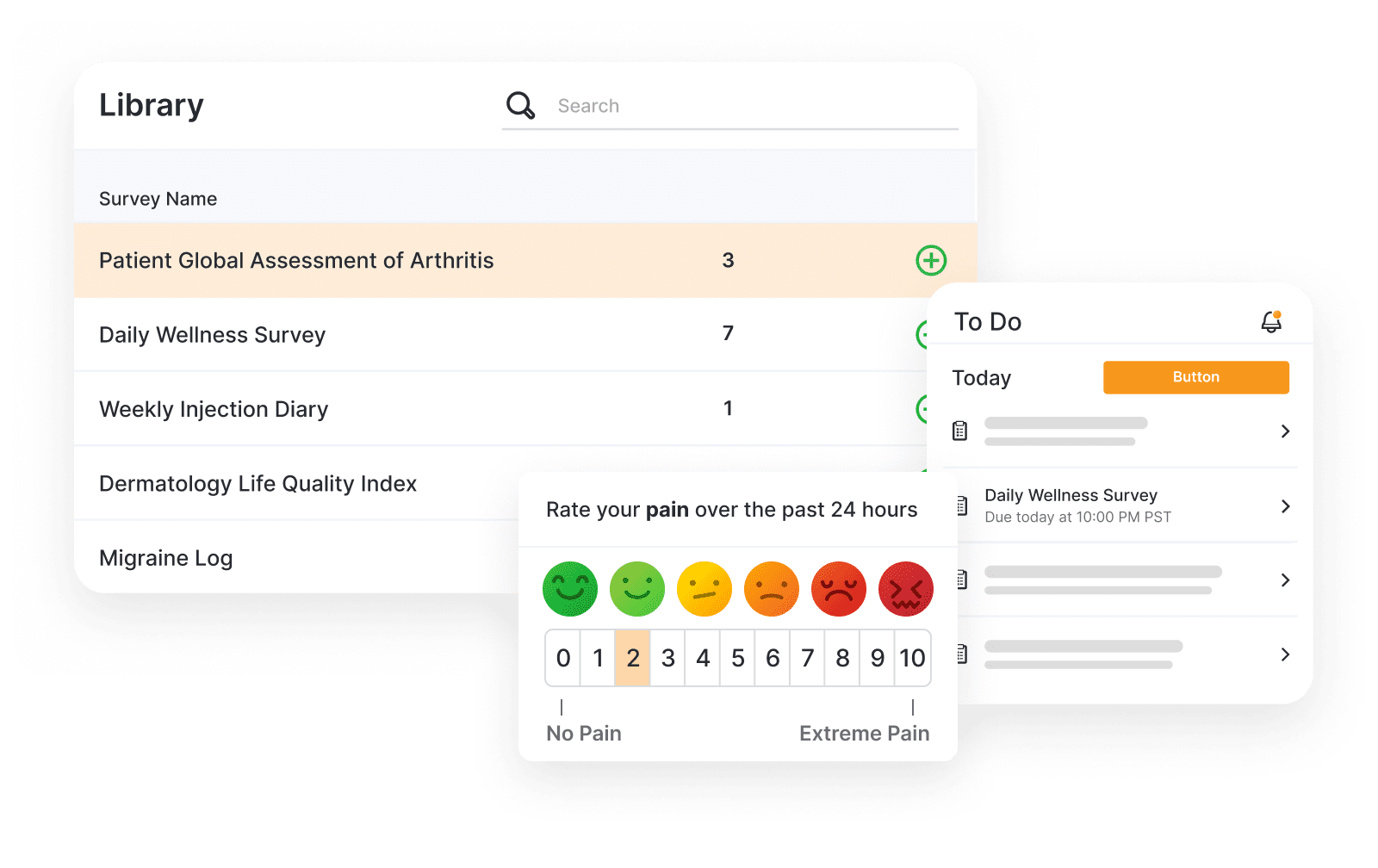
Veeva eCOA
Simplify the design, management, and completion of eCOA.
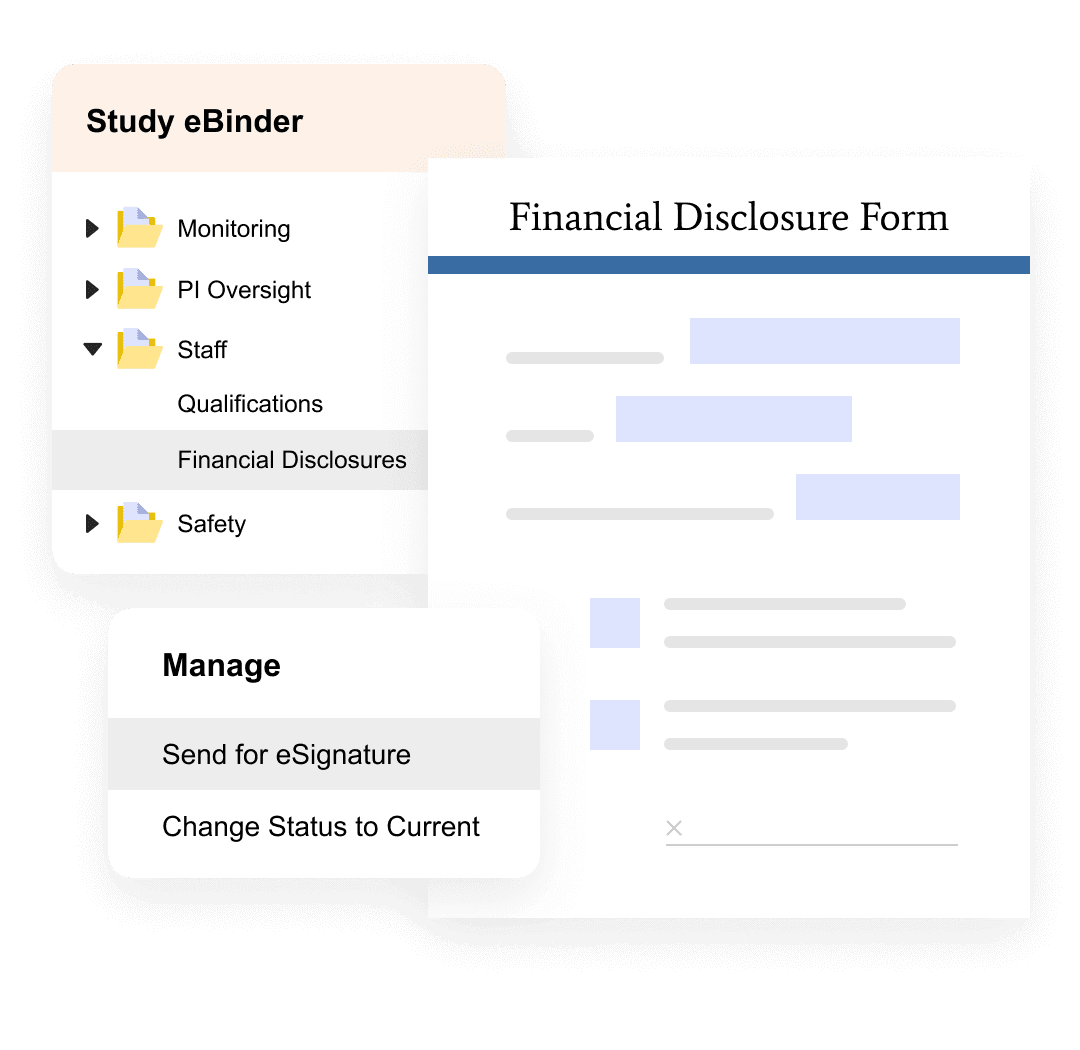
Clinical Research Sites
Simplify the site experience.
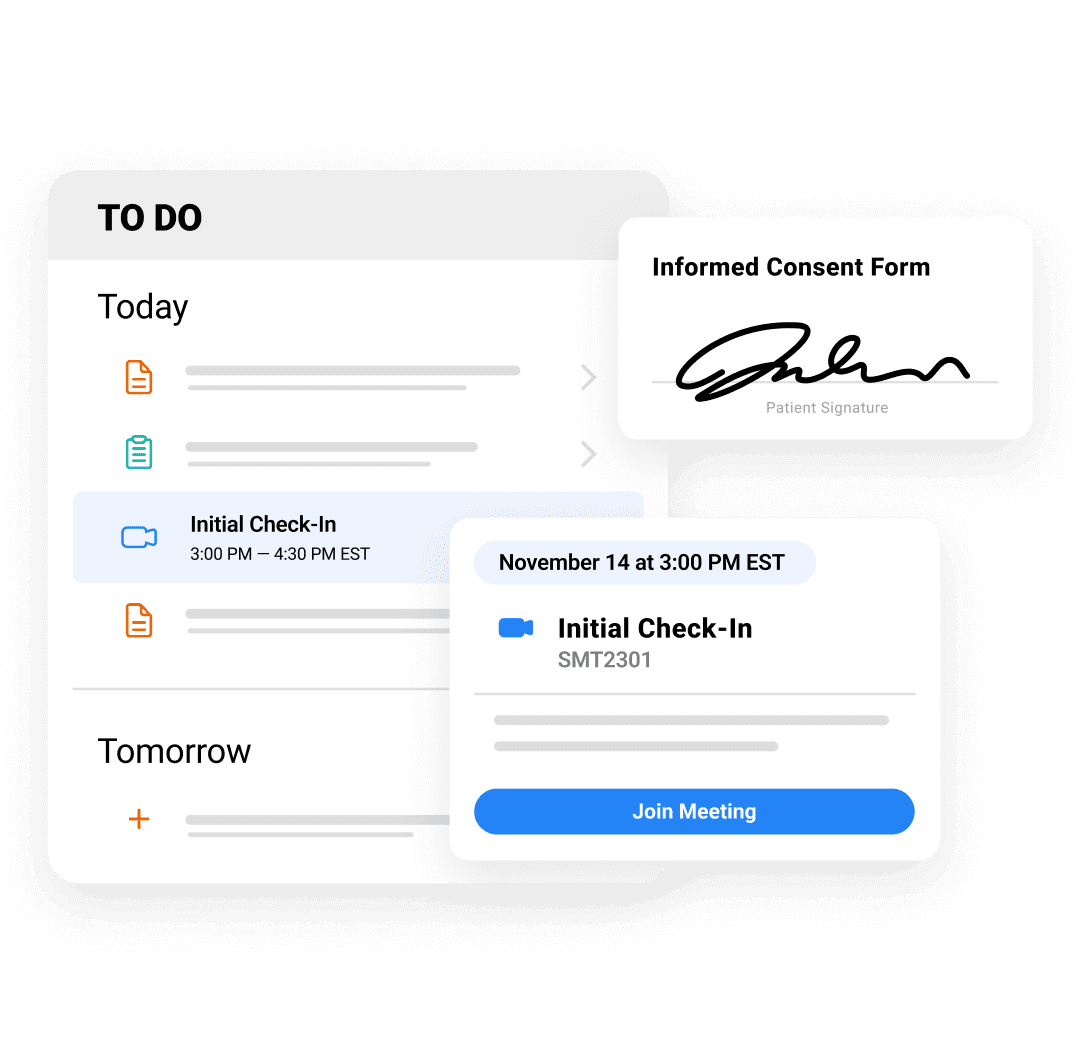
Patients
Make trial participation more accessible and convenient.
Resources
Explore and Learn
Read Guide
Explore How to Adopt a Clinical Platform

Read Customer Story
A Blueprint for Clinical Trial Transformation: Results from Three Top 20 Biopharmas

Read Customer Story
Driving Transformation with a Connected Clinical Platform

Watch Demo
See Data Flow Across the Clinical Platform

Read Report
Clinical Data Industry Research

Watch Demo
Make Taking Part in a Clinical Trial More Accessible and Convenient




