Demo Center
Watch video demos of Veeva applications, agents, and data solutions.
RTSM - eCOA Connection

Link Key People in CRM

Quality-Safety Connection
Veeva Safety Signal

Quality Events Agents

Document Translation Agents

Reimagining Engagement with Medical Interaction in Vault CRM
Veeva HCP Access
Medical Interaction in Vault CRM
Generate Aggregate Reports with Veeva Safety Workbench
Automate PSMF Content Sharing Across Regulatory and Safety

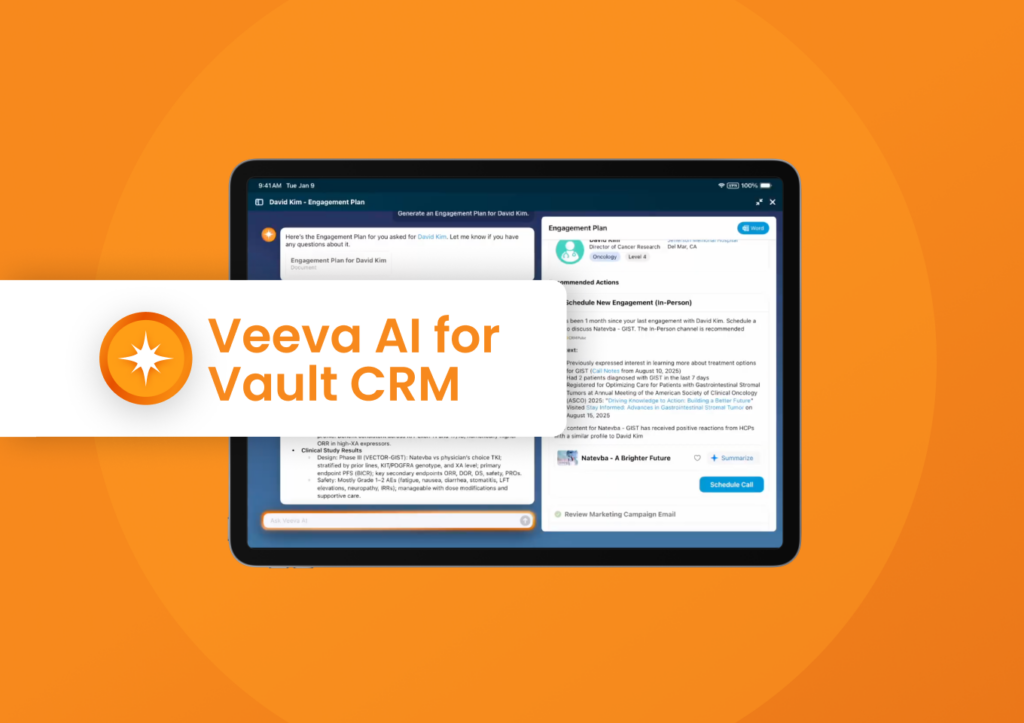
Agentic AI in Vault CRM for Deeper Insights and Interactions

CTMS Transfer Overview

OpenData Live Demo: Real-Time HCP & HCO Data
Managing the End-to-End Medical Event Lifecycle

Clinical Data Automation End-to-End

Accelerate Trials: End-to-End Flow in Clinical Operations
TMF Intake Agent and Quality Check Agent

Health Authority Interaction Agents

Case Intake Agent and Case Narrative Agent

Result Not Found
The query you've entered was not found.