Veeva eConsent
Better Inform Your Patients
with Veeva eConsent
Deliver a better site and patient experience through an
end-to-end informed consent process.
Announced 2021 Status Early Customers1-10
Overview
Patient-centric eConsent
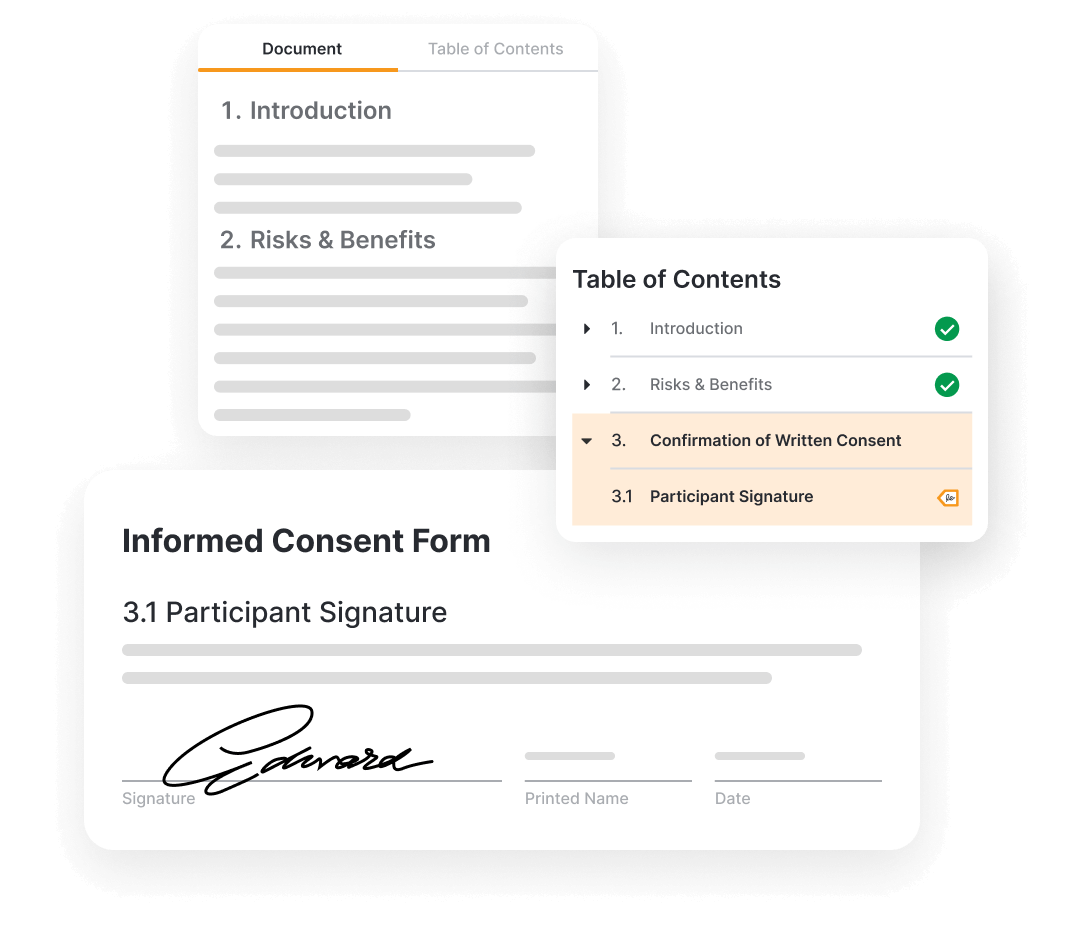
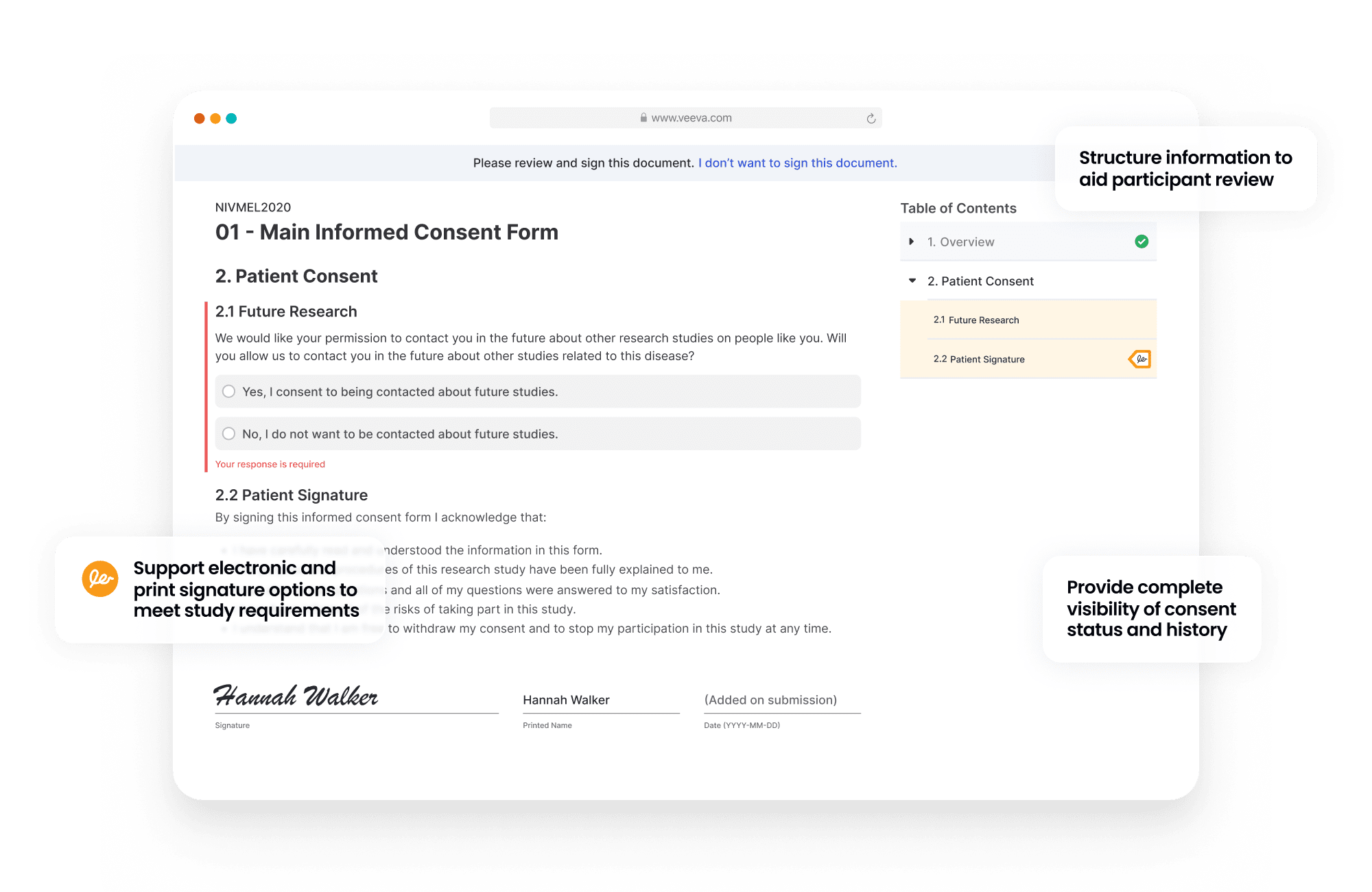
Veeva eConsent provides a digital way to consent clinical trial participants in-person or remotely.
Sponsors manage the consent forms in Vault. They can be authored in Word and digitized to include interactive elements like videos, questions, and signatures. Blinded consent data can also be viewed through the sponsors/CRO vault.
Sites are able to review and update consent documents, view participant consent status, and countersign the consents.
Participants, or other signatories consent via MyVeeva for Patients (native application or web). This is also used to access study documents and complete multiple study activities, including ePRO and visit management.