Richter BioLogics Drives Efficiency and Compliance with Unified Quality
Increased capacity equivalent to four team members
Unified quality a competitive advantage for winning business
Standardized quality management, documents, and training across sites
Streamlined reporting for real-time customer insights
A few years ago, following robust growth, Richter BioLogics faced a choice: it could either continue managing quality operations through manual, paper-based processes or it could seek to standardize site execution.
The CDMO decided to modernize and adopted Veeva Quality Cloud to enable growth, improve compliance, and deliver more customer value. Through its partnership with Veeva, Richter BioLogics has unified quality management, documents, and training on a single platform. Modernizing quality in this way has freed up capacity equivalent to four full-time team members for strategic work.
Equipped with systems that are able to scale with business needs, Richter BioLogics found that production increased threefold after it opened a new manufacturing site in Bovenau, Germany, in 2024. Critically, expansion did not entail more paper. Customer confidence is also growing, providing a competitive edge that helps to win business.
Partnering across the product lifecycle
Partnering with companies ranging from small biotechs to global biopharmas, Richter BioLogics provides support as products move from clinical development to commercial manufacturing.
Paper-based workflows introduce risks to efficiency and compliance when serving a diverse customer base. Inspections, which are carried out both by local authorities and international bodies such as the FDA and ANVISA, add to the pressure. Birte Mueller, team lead GMP compliance at Richter BioLogics, and her team realized that laborious processes involving manual handovers between sites was not a scalable approach long term.
Everyday frictions slow down manufacturing. Given its ambitions to scale manufacturing capacity, Mueller knew this would eventually constrain the business. “If we had a deviation and an ongoing failure investigation, we had to scan the paper, then email it. Colleagues at our Hamburg site would print it out and scan it again before we printed it again to have it on paper,” she says.
Complex processes also create reporting and visibility challenges for CDMOs. Each customer has a unique set of reporting and communication demands, making it difficult to manage efficiently. “Some customers want to be informed when the deviation occurs, some want to be informed every week about the status of quality events, and others only want to be informed once a month or per batch,” explains Mueller.
A manual approach to document management and training reduced capacity for higher value work. The lack of traceability during training also posed a potential compliance risk by making it difficult to monitor training status.
Modernizing quality increases customer trust
To ensure efficiency and compliance, Richter BioLogics adopted a digital-first approach to quality processes, documents, and training.
The partnership with Veeva began in 2019 with Veeva QMS, which was initially implemented to manage deviations, change controls, audits, and CAPAs. In 2023, building on this success, the company made a strategic decision to implement Veeva QualityDocs and Veeva Training.
In choosing Veeva Quality Cloud, Richter BioLogics felt confident it was using a platform trusted by regulatory authorities and customers, as demonstrated by positive feedback during inspections and site visits.
“Some potential customers at one of our sites asked us about our quality system. After they heard we use Veeva Quality Cloud, they said ‘Okay, we don’t need to talk about the quality stuff anymore, we can talk about the manufacturing’. This is a real benefit for CDMOs,” Mueller explains.
Since then, Richter BioLogics has significantly reduced its reliance on paper: opening a new site in Bovenau led to three times more production without entailing three times more paper. Managing a single platform has lessened the maintenance burden since all quality systems are connected and use the same metadata.
More time for strategic work
By unifying quality systems, Richter BioLogics has achieved significant productivity gains. Eliminating manual work and trackers has increased capacity equivalent to four full-time team members and freed time to focus on higher-value tasks that directly impact company growth.
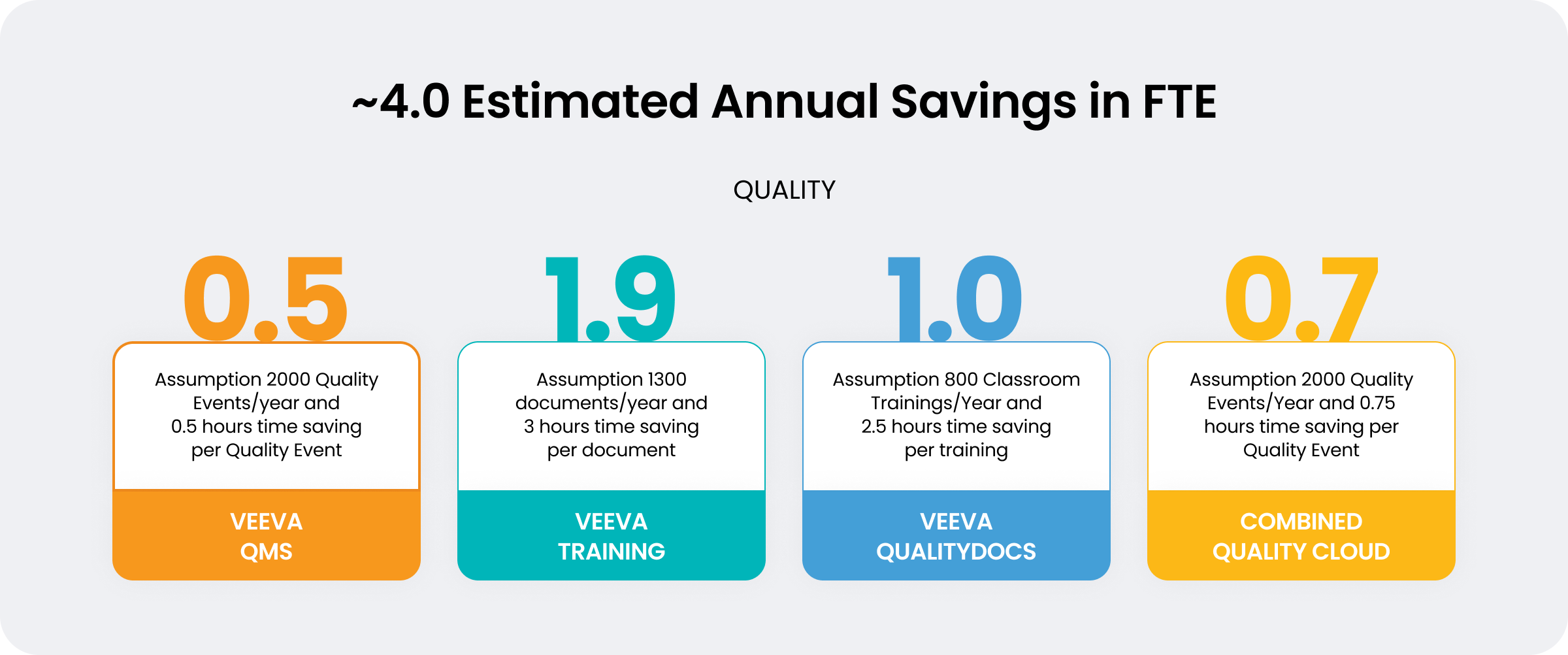
Using Veeva QMS, Richter BioLogics has streamlined previously manual processes (such as deviations), which improves audit readiness and avoids unnecessary delays during the manufacturing process. Because the team benefits from real-time status on quality events, it’s becoming easier to address customer requests using pre-defined reports. Simplified reporting also streamlines batch release preparations and boosts overall compliance.
A quick transition to Veeva QualityDocs resulted in minimal business impact. In just a few days, the team migrated over 5,000 documents to Veeva Quality Cloud. To make it easier to update templates as the company scales its manufacturing, Richter BioLogics follows Veeva industry best practices and uses out-of-the-box workflows instead of customization.
Moving 400 people from a paper-based model to Veeva Training presented a steeper learning curve. However, the company is already seeing tangible benefits. “We now have defined training metrics and can report on different kinds of topics, such as who is trained in a specific record or the training status of the core team. This makes completing the checks during batch record review much easier,” says Mueller.
Deeper partnerships through external collaboration
Richter BioLogics is planning to introduce external collaboration capabilities as the next phase of its quality transformation. Sponsors will gain direct access to its quality system, improving real-time collaboration, communication, and compliance throughout the manufacturing process.
Stronger partnerships will ultimately deliver more value to the industry. For Mueller and her team, that is what matters most.
Discover how contract services organizations benefit from Veeva Quality Cloud
More Customer Stories
Explore and learn more
Watch Video
Delivering Greater Customer Value Across Sites with Unified Quality Operations

Read Customer Story
Almac Clinical Services: Transforming Quality Processes & Collaboration with Veeva QMS

Read Customer Story
Forge Boosts Efficiency and Compliance During External Collaboration

