Blog
Lucky CRAs. Getting Automated To-do Lists for Targeted SDV
Nov 20, 2019 | Drew Garty
Nov 20, 2019 | Drew Garty
Data managers at Lotus Clinical Research used to spend hours each week building reports to identify the patients and forms that needed Source Data Verification (SDV) at the next site monitoring visit.
Risk-based monitoring solutions will tell you where to focus, but virtually all EDCs take that information and simply flag the designated fields within each respective case report form. The burden is on the CRA to visually scan every form and item, looking to see what’s flagged for SDV.
This poor usability results from a structural limitation. Traditional EDC architectures are based on case report forms, which made sense in the late 1990s when moving from paper but now constrains how the data are displayed. Regardless of role, everyone looks at data within forms.
To tackle the problem, Veeva Vault EDC introduced QuickView, a dynamic interface that optimizes the display of data by function, allowing users to focus on what matters most. With QuickView, the listings previously created by the data managers at Lotus are now automatically generated within the system itself.
Andrea Krueger, one of the lead data managers for Lotus explained, “I used to send reports to all the CRAs before they went on monitoring visits. With Vault EDC, they told me not to send reports anymore, because when they go into QuickView and click on a subject casebook, it already shows what requires SDV and what queries need to be addressed.” [Hear how else Vault CDMS is saving time for data managers at Lotus.]
A Dynamic Interface Optimized for RBM
For many organizations, “what matters” is driven by a risk-based approach. Monitors will focus their attention on the data that is the most important and the sites that have the highest risk of problems. A site with a track-record of high quality and few errors, would go through less stringent monitoring than a site showing more variation and discrepancies.
We capture your risk-based strategies in review plans according to different risk levels. You then assign the different plans to individual sites and casebooks as needed to reflect your implementation approach.
QuickView adapts the interface for CRAs based on those review plans and the site in question. As a result, monitors only see the data specified by your risk-based strategy, everything else is filtered from view.
How it Works
- Focusing on the site: The first dimension of focus is providing a perspective specific to whatever site the CRA is visiting. Filtering on a specific site shows the CRA the different studies being conducted there, and for which she or he has monitoring responsibilities. This helps the CRA plan their visit in advance and if appropriate, review data at the site for multiple studies.
- Focusing on relevant events: Once a CRA selects a study and subject casebook, they see the casebook’s entire event schedule. Typically, not every visit or event has occurred, nor does every event have data to be reviewed. The event-level filter displays only those events with forms ready for SDV and those with queries.
- Focusing on targeted data: The item-level filter controls which fields are displayed. Rather than display every datapoint collected on a form, QuickView displays only the datapoints specified for SDV based on the site’s risk profile and datapoints with queries that require their attention. If only 40% of the fields need verification for a low-risk site, only 40% of the fields are displayed for SDV.
- Focusing on open items: As you complete a task, Vault CDMS removes the items from view. At the end of SDV for that patient visit, the page is empty. It is a visual cue that all EDC related monitoring tasks are complete.
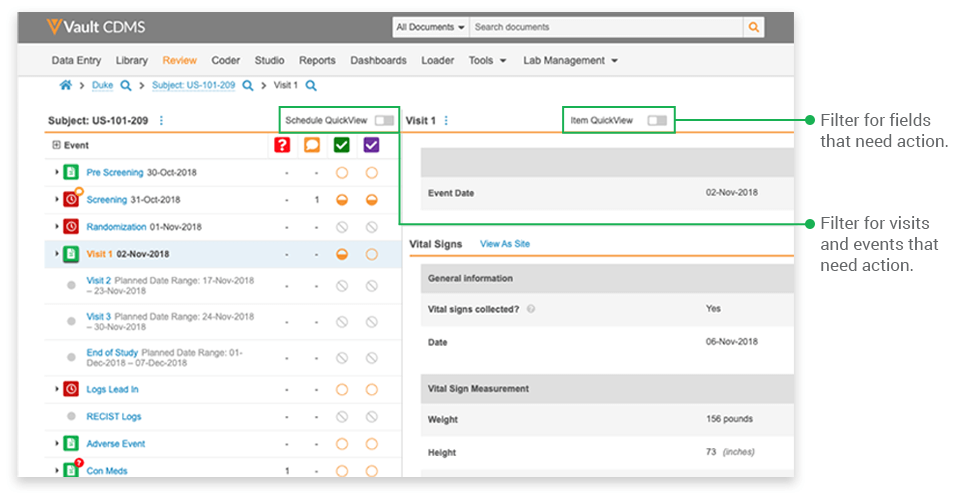
QuickView also helps monitors focus by providing a visit-centric view. All the data collected in a visit are consolidated onto a single page, organized by form. Users simply scroll down the page for an uninterrupted view of the patient’s data from that visit or a filtered view of visit data needing their attention. We all want CRAs to take a holistic approach to monitoring and to be thoughtful in their role; QuickView equips them to do so by providing a better viewpoint for the data.
Consolidating a patient’s visit data also saves time. Since traditional EDC architectures are based on case report forms (CRFs), every user gets the same form-centric view of the data. The form—rather than the business process—defines the unit of work.
In order to navigate traditional EDC systems, CRAs are forced to “turn pages” at the end of each form and wait idly while the system saves the page and loads the next. Each delay is small but adds up over time. Waiting for the “page turn” also breaks the monitor’s concentration and flow, which inherently reduces quality of thought. Each delay is a small but frequent irritation that erodes a monitor’s job satisfaction.
QuickViews in Vault CDMS will result in higher quality SDV with less time from your CRAs by listing only data that needs their attention and filtering out everything else. It is one of the many innovations we are bringing to data management.
Watch Vault CDMS’s QuickView in action here.
Drew Garty is chief technology officer for Vault CDMS at Veeva Systems.