Enhance Patient-Centricity and Improve Efficiency with SiteVault eConsent
“The continuity of having information at your fingertips and being able to answer questions instantly is invaluable.”
WHY SITEVAULT eCONSENT?

With a longstanding digital mindset, Celerion is on a journey to implement new clinical research technologies to conduct trials faster and more reliably. They want to get data to clients faster, while making it easier for participants to take part in studies. Celerion recognized early on that electronic Consent (eConsent) would increase efficiency and improve the participant experience. However, previous eConsent solutions had been unable to meet the needs of Phase I studies, which must consent and process large volumes of participants, quickly.
SiteVault eConsent offers Celerion a scalable solution for their fast-paced environment. Easy-to-use editor tools allow Celerion to create pre-approved consent templates that can be quickly adapted for specific study requirements and easily updated as study requirements change. Patients access their consent documents through MyVeeva for Patients, an easy-to-use application where they can view and complete study activities.
“eConsent has been a no-brainer for years, we just didn’t have a tool that was fast enough to work in a phase 1 environment.” — Staci McDonald, Vice President Global Scientific Clinical Operations, Celerion
THE BURDEN OF PAPER

Benefits for Participants
Celerion has received positive feedback from participants. They found it easy to access, review, and sign consents through the MyVeeva for Patients app. The documents were clearly structured, and they didn’t need to be carried around for months, unlike the old paper documents.
There were also a few unexpected surprises in the feedback. Celerion had anticipated that eliminating paper documents would benefit participants most. What they learned, however, is that patients place even higher value on other types of information shared within the MyVeeva document library. This included the study schedule and phone numbers for the nurse’s line or their study coordinator. However, the number one benefit listed by participants was having the catering menu available in the MyVeeva for Patients app. This highlights how important it is to think about participant experience as a whole, not just the aspects directly linked to study activities.
BENEFITS (PARTICIPANT RANKING)

“It was the easiest thing I did all day! I love no packet of paper to carry around with me for months. All of my consents are on my phone and easy to access.” — Study Participants
Benefits for Celerion Teams
Printing and copying were eliminated almost overnight. Paper consent processes generate huge volumes of paper, resulting in rooms full of documents and trolleys to wheel them from person to person. If a consent form was misplaced, considerable time was wasted searching until the documents were found. Moving to eConsent changed all that, as documents are automatically filed in the system and instantly available whenever required.
Removing the need to print thousands of pages of blank ICFs (informed consent forms) simplified screening preparation. Not only has this saved time, staff also reported how quiet the room is without a copy machine constantly going, which means they can hear participants better.
The ability to review data has been a real game-changer for Celerion. Documents are available at the touch of a button. This means consents can be conducted in the morning and reviewed by the monitor in the afternoon if required. Plus, if a monitor has a question, staff can look up the answer at home, whereas previously they would have to wait until the next day to go to the data room and check the relevant documents.
Screening logs have also become much more efficient. Previously, paper logs were completed manually during screening and were prone to errors, especially dates and consent version numbers. Using SiteVault eConsent, the system automatically completes the subject number, consent signature date, and version number. Monitors can view the information in real-time through a screening log report, with new groups appended to the report as they are added.
BENEFITS

“Paper must be shuttled around from room to room. We didn’t realize how much efficiency we could gain by removing these steps.” — Staci McDonald, Vice President Global Scientific Clinical Operations, Celerion
Benefits for Monitors
Monitoring efficiency significantly increased with eConsent. Signature dates and times are always legible, and ‘overwrites’ have been eliminated. Additionally, a report displays the list of participants and their consent version number, which was previously challenging to track, especially when there are many different consent versions.
Most importantly, the number of days monitors spend on-site has reduced as consent forms can be reviewed ahead of time. This means only two hours may be required to check critical documents, instead of the previous eight, which benefits monitors and their host coordinators.
IMPRESSIVE VOLUMES AFTER ONE YEAR
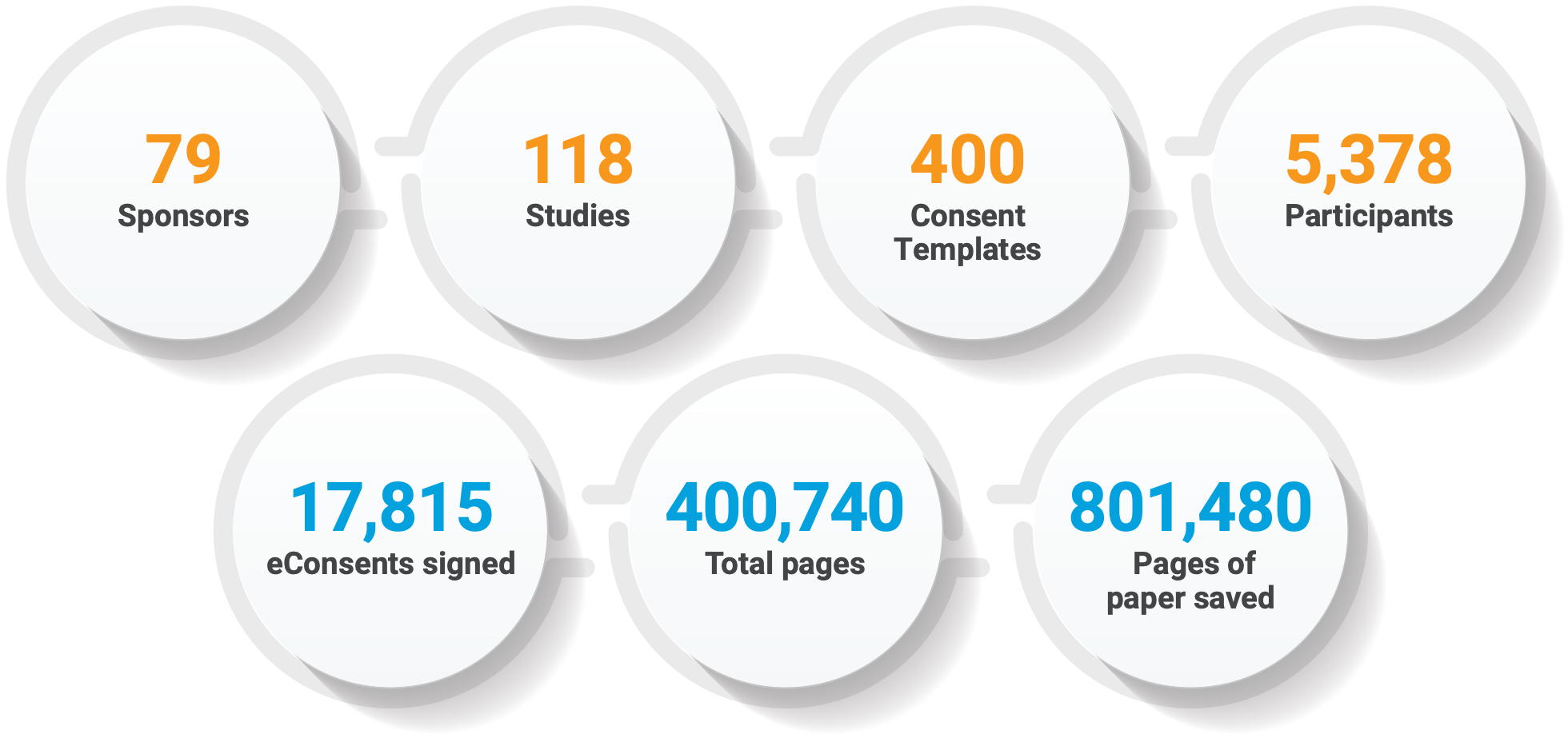
“The biggest benefit for monitors and for us is that onsite monitoring is 100% not necessary for eConsents.” — Staci McDonald, Vice President Global Scientific Clinical Operations, Celerion
Overall outcome
The introduction of eConsent has increased efficiencies across the consenting process. Perhaps even more
importantly, it has been well received by study teams, sites, monitors, and participants.
Celerion report the biggest benefits are as follows:
- Monitoring efficiencies have increased as consent documents can be reviewed ahead of time. Many queries that would have previously arisen from basic errors or legibility issues have been eliminated altogether.
- Calls to the Recruitment Call Center have reduced significantly as simple questions regarding schedules and phone numbers can now be easily found in the MyVeeva app.
- Electronic consents are automatically filed and can be accessed at any time. This has removed the infrequent, but resource-intensive searches that occurred if a consent document was misplaced.
- Communication with participants is more efficient. Participants can be kept up to date with schedule changes more easily, and the ability to share any document has significantly improved the experience of participating in clinical research.
BIGGEST IMPACT FOR CELERION



