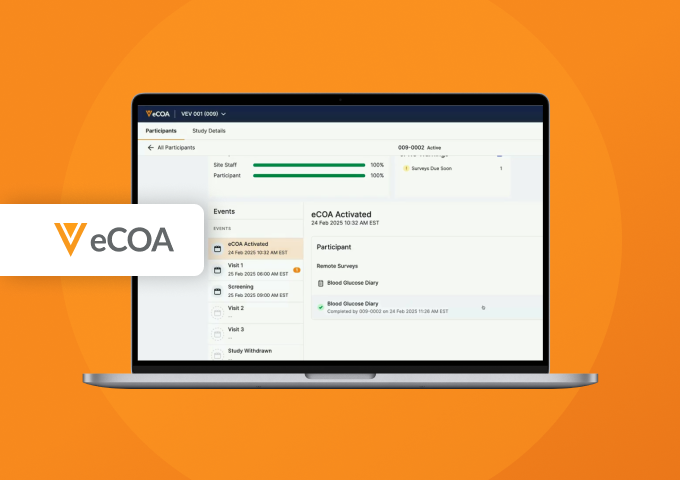
Veeva eCOA
Meet a Few of the Companies
Succeeding with Veeva eCOA

Driving Patient Engagement and ROI with a Scalable eCOA Solution
UCB’s Chief Digital Technology Officer, Edwin Erckens, discusses partnering with Veeva to scale a modern eCOA solution for patients. The partnership exceeded all original goals, achieving 100% adoption on eligible studies, ahead of schedule and delivering a strong return on investment.
“We received positive feedback from patients and site staff, and are delivering ROI, projected to save millions.”


Enabled to Self-Build, With 10 Studies Live In 6 Months
Expanding the success of the clinical platform
“With Veeva eCOA, our clinical data team can rapidly self-build studies to meet important IRB and FPI milestones while moving fast. This helps us launch eCOA quickly while improving data quality, access, and traceability.”

Why Alcon Chose Veeva eCOA
Increase Study Autonomy
Optimize Efficiencies
Increase Data Quality
Results so far:
Autonomy
Ability to self-build gives teams control over the study processes and timelines.
Highly Scalable
Optimized efficiencies and reusability have significantly shortened build times.
Data Quality
Data is accurate, complete, and attributable.
Platform Efficiencies
Seamless data flow enables data aggregation, cleaning, and transformation alongside other data sources.
200+ Validated Instruments Supported by Veeva eCOA
The Veeva eCOA Library includes 200+ fully reusable instruments, enabling faster study setup and improved data quality. With a dedicated librarian to manage the library and collaborate with authors and copyright holders, sponsors gain access to validated, pre-approved assessments that streamline deployment and minimize delays.
View the full list of instruments in the Veeva eCOA Library.

Members of the Electronic Clinical Outcome Assessment (eCOA) Consortium
Veeva has joined the Critical Path Institute (C-Path)’s eCOA Consortium to collaborate with industry leaders on improving eCOA standards for all.
The consortium provides scientific leadership and best practice recommendations for the use of electronic data capture technologies and services in clinical trials.

“C-Path’s eCOA Consortium is the only dedicated pre-competitive alliance of eCOA/DHT technology and allied service providers in existence. Membership of the eCOA Consortium conveys a clear commitment to driving the effective use of eCOA & DHT systems in clinical research. I’m absolutely delighted to have Veeva amongst our membership to help the eCOA Consortium and the industry as a whole move forward.”