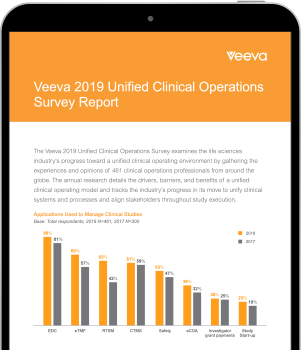
See new results from one of the industry's largest, global clinical operations surveys.
Findings show sponsors and CROs are making progress in unifying clinical systems, streamlining end-to-end processes, and aligning stakeholders to improve trial performance.
The report includes research and analysis on the following topics:
- Drivers and barriers to unifying clinical systems and processes
- Information exchange in clinical trials
- Accelerating study start-up
- Active TMF management and oversight
- Improving clinical trial operations