Veeva Submissions Archive
Dynamic Search, Filtering, and Navigation
Submissions Archive is a global, secure repository of submission published output. It functions as the authoritative source of applications submitted to health authorities.
Submissions Archive includes a viewer that supports all electronic and paper formats, with PDF link navigation provided for electronic formats. Users can view submissions and health authority correspondence alongside all previously submitted applications.
The embedded document viewer provides visibility into each document in the structure.
The Active Dossier feature displays the submission components that are currently active for any product / market combination.
Announced 2015 Status Mature Customers 100+
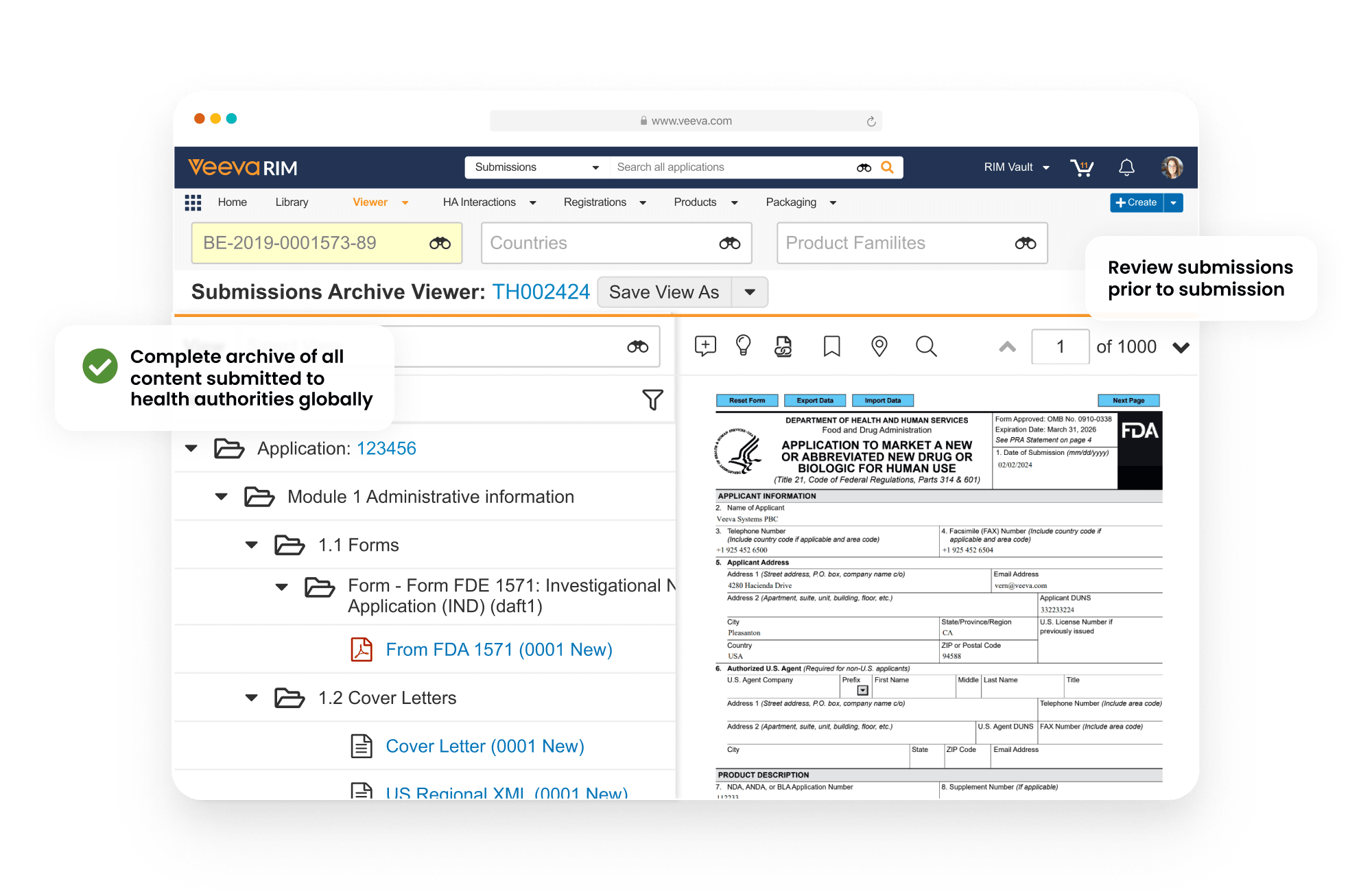
Maintain a list of current documents for a given product and market with Active Dossier
Overview
Dynamic Search, Filtering, and Navigation

Submissions Archive is a global, secure repository of submission published output. It functions as the authoritative source of applications submitted to health authorities.
Submissions Archive includes a viewer that supports all electronic and paper formats, with PDF link navigation provided for electronic formats. Users can view submissions and health authority correspondence alongside all previously submitted applications.
The embedded document viewer provides visibility into each document in the structure.
The Active Dossier feature displays the submission components that are currently active for any product / market combination.
Veeva AI for Regulatory
Health Authority Interactions Agent
Automates HA interactions for faster simultaneous approvals globally.
Application Assistant Agent
Conversational insights into regulatory activities with clear and consistent narratives.
Impact
Exceed the likely outcome
15
of the top 20 companies use Veeva RIM
88
IT systems consolidated into one
90%
reduction in written standards
Why Veeva Submissions Archive
Dynamic search, filtering, and navigation
Customer Success
Veeva RIM is trusted by 400+ top
and emerging biopharmas