Features Brief
Veeva Development Cloud Features Brief
Technology Foundation for Product Development
Veeva Development Cloud is the technology foundation for product development that brings together applications for clinical, regulatory, and safety to help organizations drive end to-end business processes. Today, product development systems are not well integrated, which creates inefficiencies and slows down critical operations. Veeva is the first and only company to offer unified suites of applications that are connected on a single cloud platform. This enables organizations to centralize content and data across global departments for greater efficiency and compliance.
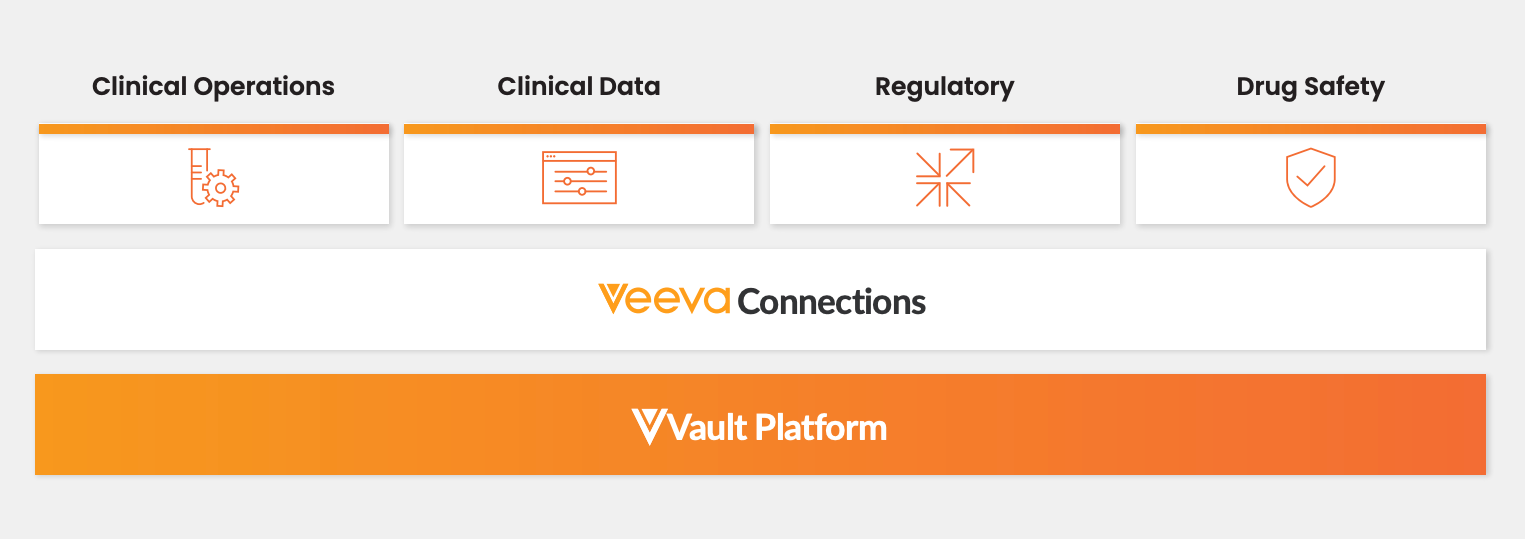
Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents across clinical, regulatory, and safety Vaults. Designed to streamline cross-functional business processes, the Connections break down silos and provide greater visibility, and automate manual tasks. View the Veeva Connections Resource Hub for a full list of available Veeva Connections.
Veeva Clinical Operations
Simplify and standardize clinical trial execution.
Veeva Clinical Operations unifies clinical systems and processes on a single cloud platform to enable end-to-end trial management.
- Veeva eTMF – Enable active eTMF for real-time inspection readiness.
- Veeva CTMS – Enable proactive trial management.
- Veeva Payments – Pay clinical research sites faster.
- Veeva Study Startup – Accelerate time to site activation.
- Veeva RTSM – Randomize subjects and manage trial product supply.
- Veeva Site Connect – Automate information sharing.
- Veeva Study Training – Streamline and automate training.
- Veeva Disclosures – Centralize clinical trial disclosures.
- Veeva OpenData Clinical – Provide investigator and site data.
Veeva Clinical Data
Accelerate study timelines with modern, innovative applications for clinical data.
Veeva Clinical Data helps clinical teams collect, aggregate, clean, and manage trial data with agility and speed.
- Veeva EDC – Collect, clean, and review study data.
- Veeva CDB – Manage complete and concurrent study data.
- Veeva eCOA – Capture responses directly from clinical trial participants.
Veeva RIM
Bring speed and agility to your regulatory team with unified RIM.
Unify regulatory systems and processes on a single cloud platform for end-to-end submission and registration management.
- Veeva Registrations – Track global registrations.
- Veeva Submissions – Plan and manage submissions.
- Veeva Submissions Publishing – Publish to health authorities.
- Veeva Submissions Archive – Access submissions history.
Veeva Safety
Safety suite of applications operate as a unified pharmacovigilance system on a single cloud platform to maximize operational efficiencies and improve patient safety.
- Veeva Safety – Real-time management and oversight for adverse events.
- Veeva SafetyDocs – Centrally manage pharmacovigilance content.
- Veeva Safety Workbench – Run advanced reports, queries, and analytics.
- Veeva Safety Signal – Reliably detect, validate, and manage potential safety signals.
To learn more, watch this 13 minute demo of the Veeva Development Cloud in action.