Features Brief
Veeva LIMS Features Brief
Modernized Quality Control With Veeva LIMS
Veeva LIMS enables Quality Control to optimize batch release testing, stability study management, and environmental monitoring. It drives detailed sample management, digital test method execution, specification adherence, and review by exception to accelerate the release of product.
LIMS promotes compliance by verifying user qualifications from Veeva Training, displaying effective test method procedures from Veeva QualityDocs, and initiating quality events directly in Veeva QMS, ensuring proper resolution prior to batch disposition.
Business Benefits
-
Embrace digital-first
Replace legacy systems and paper processes to increase reliability and accuracy with one unified solution.
-
Modernize QC processes
Streamline end-to-end QC data management and test execution processes to break down silos across systems, teams, and data.
-
Unify the quality ecosystem
Reduce errors and improve speed with seamless quality processes and automated workflows.
Features
-
QC Batch Disposition
Manage the end-to-end QC Batch disposition workflow, including batch definition, QC sample management, test assignment and execution, specification evaluation, multi-level review by exception, generation of the Certificate of Analysis (COA) and publication of outcomes to Vault Batch Release.
-
Stability Study Management
Design, execute, and oversee stability studies with inventory and time point pull management, test assignment and execution, specification evaluation, multi-level review, and generation of time point and study summary reports.
-
Sample Management
Track samples from collection to storage and receipt in the lab. Print labels and maintain current location.
-
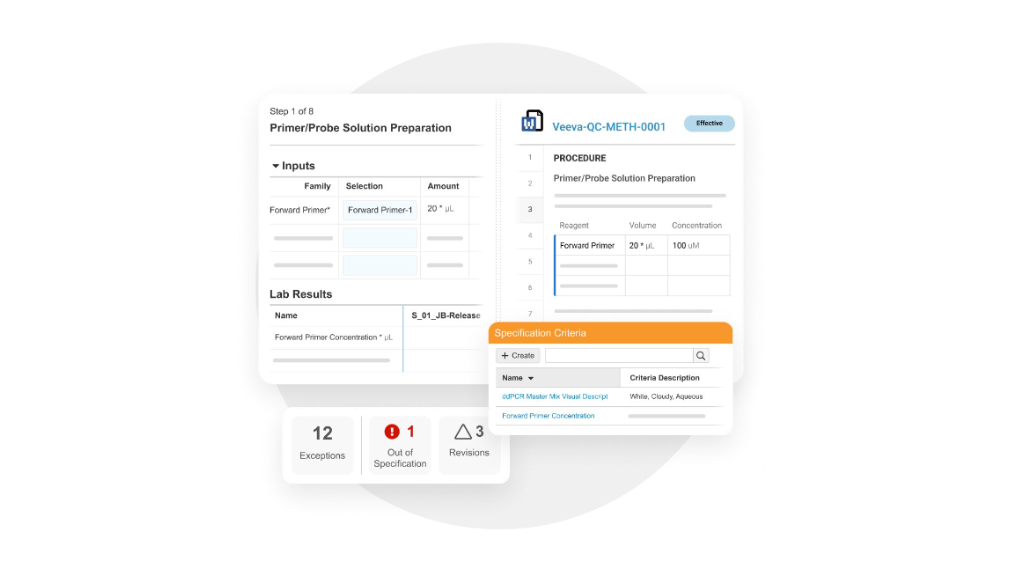
Digital Method Execution
Perform digital method execution following the effective test method procedure rendered on screen, ensure compliant usage of instruments, equipment, and consumable inventory, retrieve data from instruments, and perform calculations.
-
LIMS Master Data Change Management
Reduce administrative burden and cost, and improve change control execution by centralizing the management of LIMS changes. LIMS automates the identification of records related to a change and facilitates accurate up versioning of impacted data objects to reduce the impact of business changes on the QC lab.
About Veeva Quality Cloud
Veeva Quality Cloud accelerates the manufacturing of high-quality products to a greater number of patients. The cloud platform unifies applications, processes, and partners across content management, training, quality management systems (QMS), and QC lab solutions (LIMS).