Features Brief
Veeva Submissions Publishing
Continuous publishing on a unified RIM platform

Traditional publishing processing activities are sequential and disjointed. Companies often rely on multiple technologies including document management systems, publishing tools, and spreadsheets for planning and tracking submissions. Each gap between systems introduces inefficiencies and delays.
Veeva Submissions Publishing is a continuous publishing solution within the Veeva RIM Platform that dramatically speeds up submission delivery. It eliminates the need for standalone publishing software and manual document exchanges between fragmented systems. Users can complete publishing tasks, including cross-document hyperlinking and validation, earlier in the process when issues are easier to fix. This avoids multiple rounds of unnecessary internal reviews.
Used in conjunction with Veeva Submissions and Veeva Submissions Archive, Veeva Submissions Publishing streamlines end-to-end publishing processes to drive greater automation, transparency, and speed.
Business Benefits
-
Faster submission preparation process.
Publishing in parallel with authoring while consolidating and automating workflows eliminates redundant steps.
-
Better control and end-to-end view.
Authors and publishers collaborate side-by- side in the same tool with a holistic, consistent view of the entire process.
-
Trusted quality.
Viewing documents in their final context while they are still in draft reduces the risk of errors and content re-work.
-
Unified RIM.
Connect end-to-end regulatory processes and improve efficiency as part of the Veeva RIM Platform.
Features
-
Assisted Submission Building
Eliminate manual steps with rule-based auto-matching. Leverage documents and metadata from submission content plans.
-
Submission Independent Hyperlinking
Create hyperlinks that are independent of the submission structure so they can be used earlier in the process during document reviews and reused across multiple submissions.
-
Background Validation
Automate validation and link testing with a tool that identifies problems as submissions are being built.
-
Submission-ready Rendering
Automatically render all documents with the correct PDF standards. Navigate web links, cross-references, and tables of contents directly from the viewer.
-
Real-time Status Reporting
Gain visibility into the complete submission process including submission status and individual document readiness.
-
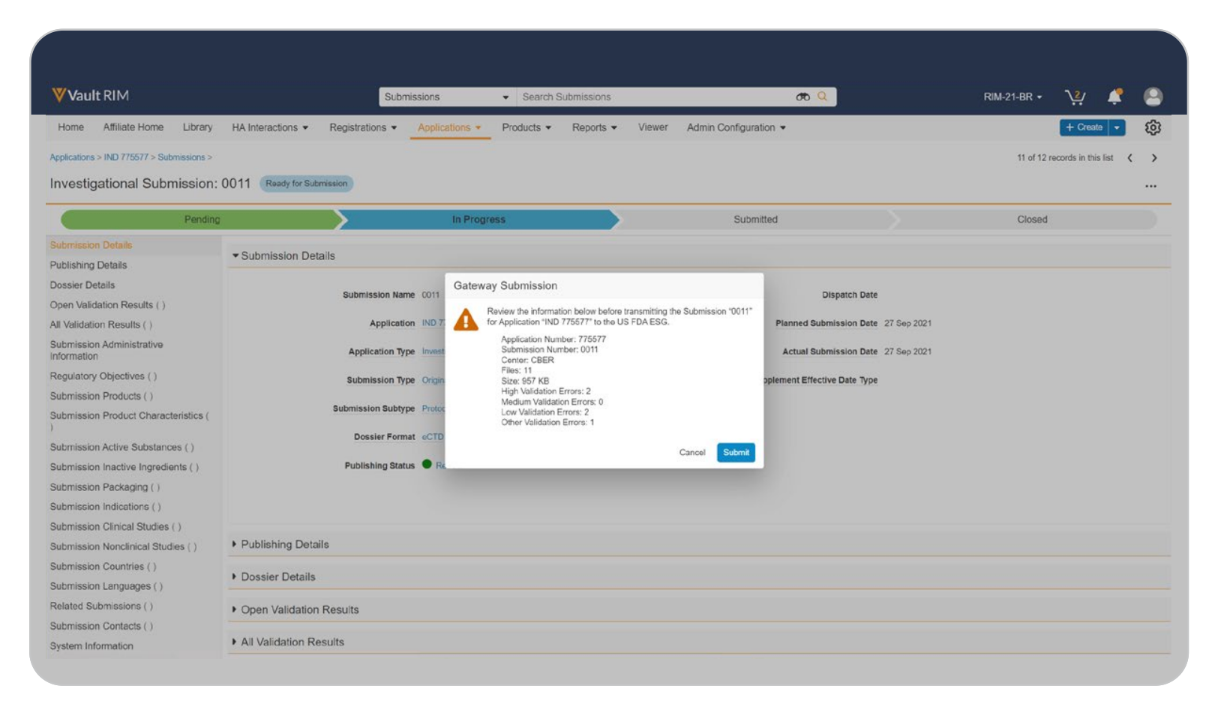
Gateway Integration
Transfer submissions through health authority electronic submission gateways, automatically archiving all gateway receipts and responses.
-
eCTD Support
Stay compliant with global eCTD and non- eCTD standards.
-
Granular Document Merging
Merge submission-ready documents across an entire content plan or part of the content plan to meet agency requirements.
Veeva RIM
Veeva Submissions Publishing is part of Veeva RIM, which streamlines global regulatory processes on a single, cloud-based platform. This enables life sciences companies to:
- Ensure teams are developing reliable regulatory content with high data integrity
- Coordinate regulatory efforts across headquarters, affiliates, and partners
- Respond faster to changing regulations
- Increase end-to-end process efficiency from submission planning to publishing