Features Brief
Veeva Submissions Archive Features Brief
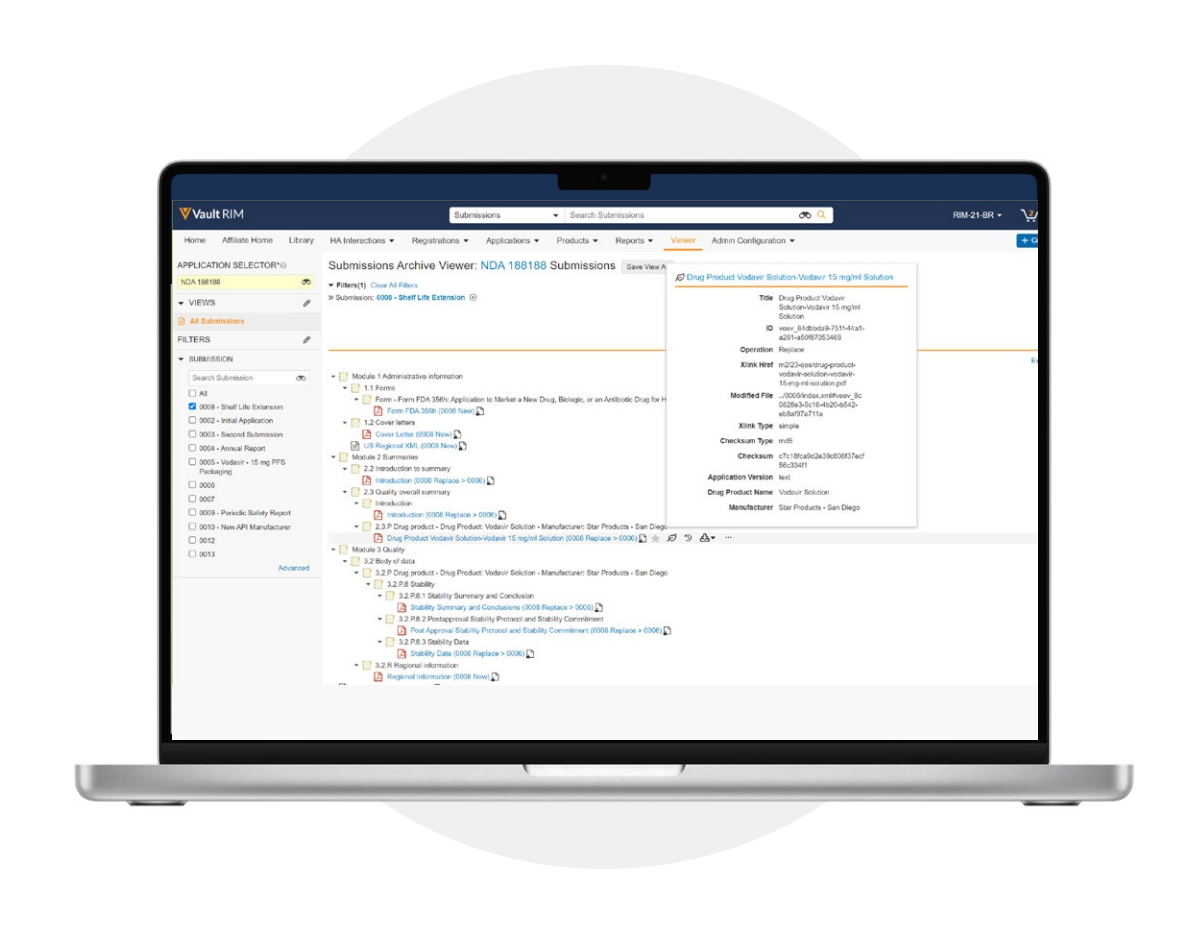
Complete history of regulatory submissions

Regulatory teams often use a patchwork of systems to track historical submissions, creating confusion and slowing responses to health authorities. Veeva Submissions Archive makes it easy to find the right information in a validated cloud environment.
Regulatory teams can store eCTD and non-eCTD electronic submissions (NeeS) and link health authority correspondence to related submissions for a complete view of regulatory communications. Affiliates can download submissions or submission components for reuse in local markets and upload submissions already sent to various health authorities.
Veeva Submissions Archive enables companies to import submissions directly from file shares while preserving the eCTD XML backbone, folder structure, and interdocument hyperlinks. Users can navigate documents exactly as they were submitted to regulatory agencies and directly from the repository without needing to download files. An integrated eCTD viewer provides current, sequential, cumulative, and regulatory action views so users can quickly see a submission’s full lifecycle.
Business Benefits
-
Increased visibility.
Comprehensive view reduces time and effort to find and access submission information.
-
Faster decision-making.
Advanced search capabilities help users easily locate information, accelerating timelines.
-
Improved access.
Spend less time maintaining compliance and reallocate time to more strategic tasks.
-
Unified RIM.
Connect end-to-end regulatory processes and improve efficiency as part of the Veeva RIM Platform.
Features
-
eCTD and Non-eCTD Import
Import final submission packages for your records and future reference. Timely updates ensure regulatory compliance in alignment with regional requirements.
-
Integrated Viewer
Leverage an integrated, cloud-based viewer for eCTD, NeeS, and legacy submission formats, which reduces the number of tools on the regulatory desktop. For eCTD submissions, view and filter XML values to focus on dossier reviews.
-
PDF Link Navigation
Navigate PDF hyperlinks across documents within a submission, across submissions, and even across applications. There’s no need for separate tools, file shares, or downloading files.
-
Full Lifecycle Viewing
View the complete dossier lifecycle with current, sequential, and regulatory action views. See cumulative changes for each document.
-
Health Authority Interactions
Retain and classify all correspondence with health authorities. View correspondence documents in the context of the application and associated submission.
-
Dynamic Access Control
Use rule-based access control to dynamically calculate permissions that ensure people can see only what they need and nothing else.
-
De-duplicate Documents
Store documents used in multiple submissions once and only once with the correct leaf details and metadata for each submission.
-
Smarter Navigation Display
Access a document’s complete history of lifecycle operations. See where a document is used, including direct links to each submission.
-
Bulk Submission Export
Quickly export multiple submissions to support product divestitures, collaboration, and outsourced publishing.
Veeva RIM
Veeva Submissions Archive is part of Veeva RIM, which streamlines global regulatory processes on a single, cloud-based platform. This enables life sciences companies to:
- Ensure teams are developing reliable regulatory content with high data integrity
- Coordinate regulatory efforts across headquarters, affiliates, and partners
- Respond faster to changing regulations
- Increase end-to-end process efficiency from submission planning to publishing