Blog
Paper as a Source in EDC: A High Price for Low Quality Data
Aug 09, 2017 | Hugo Cervantes
Aug 09, 2017 | Hugo Cervantes
Technology has transformed almost every aspect of our daily lives, yet site coordinators and clinical research personnel still rely on pen and paper to record patient visits before transcribing into EDC. Considering smart devices are nearly a decade old, it’s remarkable that critical patient information is first recorded onto paper rather than captured digitally.
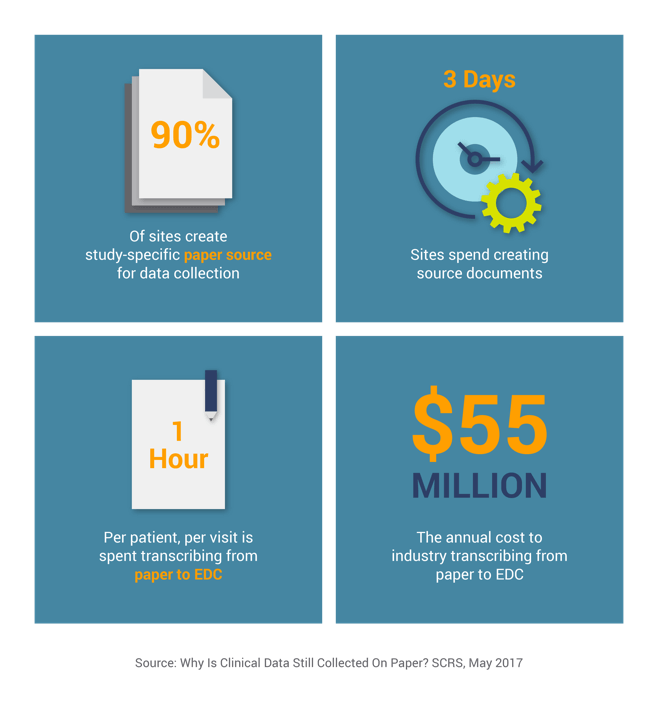
The above estimates do not even include the cost of correcting the data, which is often incorrectly transcribed or not collected per the protocol. They also don’t consider the cost of onsite monitoring and source data verification (SDV), the process that checks the accuracy of the transcription. Onsite monitoring and SDV typically consume 14% of a study’s total budget1, increasing the costs from paper data collection many times over.
Why hasn’t eSource taken off?
These many challenges, combined with the advancements in technology and increasing regulatory focus, naturally lead to electronic source (eSource) as the solution. Per the FDA, eSource is defined as “data initially recorded in electronic format…can include information in original records and certified copies of original records of clinical findings, observations, or other activities captured prior to or during a clinical investigation…”
eSource technology addresses data quality and productivity challenges by eliminating paper source and allowing sites to electronically capture and submit patient source data. Sites no longer have to spend days creating their own paper source documents, nor transcribe the collected data into EDC. eSource drives higher quality data through real-time data validation, preventing queries that typically occur when data is transcribed into an EDC application and the data is immediately available for review. Despite such benefits, eSource usage is still low and it has only been utilized in a fraction of studies.
This lack of adoption is even harder to understand when regulators have in recent years encouraged eSource adoption. The FDA promotes moving away from paper source in its 2013 electronic source guidance. Their final guidance on electronic source data in clinical investigations recommends elimination of “shadow CRFs.” The 2016 integrated addendum to ICH E6 also incorporates various approaches based on electronic data recording such as certified copies, ALCOA (attributable, legible, contemporaneous, original, accurate), and centralized monitoring.
So why is eSource adoption still so low? The biggest obstacle is that it is still very difficult to run an eSource-only study and in many studies, EDC is still required. In these situations, organizations have to work with separate EDC and eSource technologies. This immediately doubles the overhead: another software license, managing the design, build and validation of an additional application, trying to integrate these two disparate systems, and often provisioning device hardware for this additional eSource application. At the end of all this, study teams cannot expect real-time data access between two disparate systems.
eSource & EDC combined in a cloud platform
eSource and EDC need to be combined in a single platform to successfully drive the industry’s move away from paper as source. A combined platform allows study teams to perform just one design, build and validation for both EDC and eSource. This single build automatically generates tailored interfaces and workflows for sites, monitors and data managers. Study data is then accessible across the platform for instant review and decision-making without any integrations required.
Modern eSource and EDC combined should enable study builds in days, not weeks, through features such as simple ‘drag and drop’ user interfaces that remove the need for technical skills and programming. Review and approval times can be cut using configurable workflows using triggers and notifications to prompt activities – also giving project managers immediate visibility into any bottlenecks. The output is a dedicated user experience for each consumer/contributor, not a single view prioritized for one user community (e.g. Data Managers). As every site is different, with localized practices and requirements, modern eSource should enable sites to easily customize their electronic source forms to suit their workflow – without impacting the CRF data that the sponsor receives.
Intuitive eSource CRFs, with increased data collection guidance, reduce the number of queries and drive greater site protocol compliance. The data collected in eSource is immediately available for review and monitoring in EDC. However, this elimination of paper will naturally reduce source data verification and allow monitors to either consolidate onsite visits or focus onsite time on higher value activities, such as patient recruitment.
To learn more on this topic, please join us for our recent webinar ‘EDC and eSource: Combined for Better Data and Faster Insights.’ – you can access it here.
1Clinical Trials, February 2016