Blog
Collaboration and Metrics are the Cornerstones of Optimized TMF
Mar 09, 2018 | Jason Methia
Mar 09, 2018 | Jason Methia
Collaboration and metrics utilization are critical components when it comes to optimizing your TMF. Improving collaboration between sponsors, CROs, and sites enables clinical operations professionals to work more efficiently, achieving greater results and improving visibility into the TMF. This creates an opportunity to more effectively evaluate TMF completeness, accuracy, and timeliness.
Here, we look at the evolution of collaboration and metrics utilization in TMF operating models and discuss emerging trends that will impact how the industry thinks about TMF management.
Current state of metrics and collaboration within TMF
The industry is in a state of transition, moving further away from passive, ‘back office TMF’ operating models – where TMF is transactional – to more active models, where TMF operations are thought of as part of trial execution.
In a passive TMF operating model, documents are uploaded into the system only after they are finalized. Visibility into the early progress of a document’s lifecycle and associated business processes is typically not understood; TMF is mostly regarded as separate to trial execution.
Conversely, active TMF management ensures all TMF documents, related information, and processes are managed in the same system, at the same time, as they are being executed – maintaining the TMF in a constant state of inspection readiness.
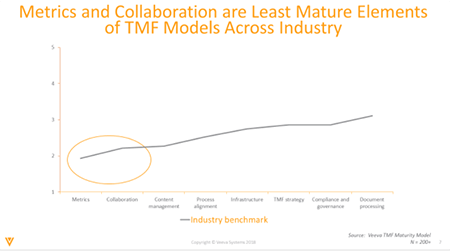
Collaboration and metrics are currently the least mature TMF elements, as shown in our Veeva TMF Maturity Model – a tool that provides standard measurement of how the industry is dealing with various TMF elements. These will mature as the industry moves to more active models, where TMF is considered critical for trial execution.
The evolution of collaboration and metrics in TMF
Historic ‘back office’ TMF models are evolving. Here, metrics are mostly about document processing times, with limited focus on TMF health. TMF is segregated from trial execution, and successful cross-party collaboration is almost impossible to achieve.
A desire to increase sponsor oversight and risk-based methods, while utilizing real-time metrics for active TMF management, is driving the industry shift from ‘back office’ to early, active TMF operating models.
In these models, TMF metrics mostly reflect quality and TMF is considered critical and monitored. There is an increased focus on collaboration, with a greater desire for direct CRO access. Additionally, some study team members use eTMF to execute. There is still an opportunity, however, to improve metrics usage and collaboration within company functions and with external partners, like sites and CROs.
Now, the industry is heading to active TMF management, where TMF operations are a fundamental part of trial execution and TMF is actively monitored as a trial function. In this model, metrics reflect both business operations and quality. Collaboration is enhanced and it’s likely most/all parties are using eTMF to execute.
Future trends for collaboration and metrics utilization within TMF
There are two trends I see emerging. First, increased system interoperability and information sharing between CROs and sponsors.
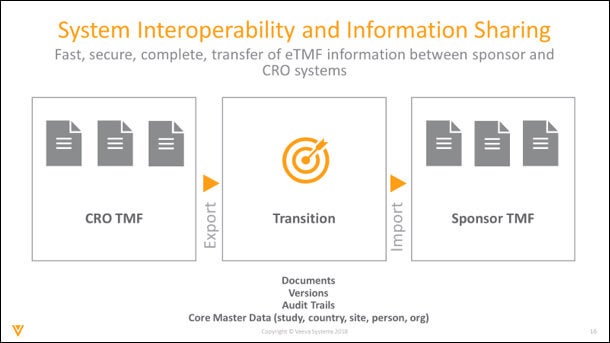
This trend would enable the moving of certain documents to be automated, rather than manual. It would aid collaboration, giving each party the autonomy to work within their own systems, without compromising partner visibility. Data housed in each system would reflect the whole business process, ensuring the collection of meaningful metrics.
Shift in site engagement
As a separate trend, we expect to see a change in how we engage with sites as part of trial execution. Site transactions today are largely manual and redundant. Sponsors and CROs duplicate technologies to execute clinical trials, which drives data and content complexity, increases costs, and hinders collaboration.
We can expect to see a change in direction, driven by sponsors and CROs’ shared desire to achieve site oversight and efficiency. Sites, meanwhile, are seeking compliance and efficiency and want systems that help with their operations.
Historically, sponsors have offered technology to sites to support transactions, but this has done little to help with process. As a result, adoption is poor and the relationship between the site and sponsor remains transactional. Like the automation of shared data from sponsors and CROs, the shift in site engagement will focus on automating the flow of shared data versus using transactional systems to share information.
We will see unified technology between sites and sponsors, where both parties have their own operational technology which works together to exchange shared documents and data automatically.
A data exchange would help simplify and automate interactions and processes, resulting in fewer transactions. Sponsors, CROs, and sites would have their own environments, with standard internal processes and efficiencies.
The data exchange would offer oversight and efficiency across partners – pushing content and data to the right environments in a predictable way. This enhanced collaboration with sites would deliver greater depth and breadth of meaningful collaboration and metrics.
How mature is your TMF? To see how you compare to the industry, take the 12-minute TMF Maturity Model evaluation here.