Blog
Driving Insights to Action
Jul 03, 2018 | Laurie Wood
Jul 03, 2018 | Laurie Wood
Highlights from Veeva’s 2018 Unified Clinical Operations Survey Presentation at #DIA2018
The DIA 2018 Global Annual Meeting held in Boston last week brought together thousands of innovators from around the globe in an open exchange of ideas, research, and trends impacting the global life sciences community. Veeva was delighted to attend the conference and participate in the many discussions and activities taking place throughout the week.
The theme of the conference – driving insight to action – was particularly apt this year as Veeva’s Michael Burton presented the findings from Veeva’s 2018 Unified Clinical Operations Survey. As one of the industry’s largest global surveys, the research found that nearly all (99%) clinical leaders report the need to unify their clinical environment, and most (87%) have a plan in place to get there. The industry sees it as essential to increasing visibility, quality, and speed of execution.
Top Drivers for Unified Clinical Operations
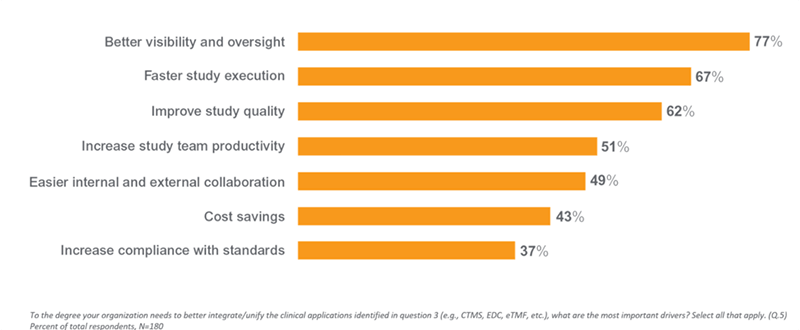
The research shows that many of the challenges organizations face today in managing clinical trials stem from the disparate nature of their processes and systems. An average of four applications are used to manage respondents’ clinical studies, and more than one-third (38%) use at least five applications. Many of these systems were implemented by functional area, creating application and process silos which have contributed to the industrywide move to unify the clinical trial landscape.
Despite these challenges, findings show the industry is advancing its systems and processes in major clinical areas such as eTMF and study start-up. As a result, trial performance is increasing at each stage of the clinical development process.
Take, for example, the movement toward active TMF management. Adoption of eTMF has grown significantly over the past four years – half of sponsors (50%) now have a purpose-built eTMF application versus 13% in 2014. This growth signals a shift away from passive TMF management toward a mature, active TMF operating model where TMF processes and information are managed in real-time. These active TMF solutions have a direct, positive impact on inspection-readiness and trial performance.
The emergence of modern systems and processes are helping to propel further change in the industry. Streamlining study start-up and leveraging purpose-built study start-up applications has become a priority focus for nearly all (83%) organizations. Clinical leaders also see improving CTMS as a way to gain greater visibility and inform proactive decision making – driving insights to action for improved trial performance.
This is an exciting time for the life sciences industry. Clinical leaders see tremendous opportunity to transform their operations by unifying their clinical environments. The change underway will enable the industry to better manage the growing complexity of trials, improve compliance, and leverage insights across the full lifecycle to accelerate time to market.
Read the full global report to gain insight and analysis on the needs, barriers, and opportunities for unifying the clinical trial process.