Blog
GSK Puts Data First in New Clinical Agility Benchmarks
Jun 15, 2023 | Richard Young
Jun 15, 2023 | Richard Young
Just as EDC did when it hit the scene almost 20 years ago, the disruption caused by COVID-19 has presented clinical data management teams with the perfect opportunity to rethink their approaches to data management.
“COVID has given us the chance, once again, to bring about disruption that will change the face of the industry over the next five to 10 years,” explained Mayank Anand, VP and global head of data strategy and management for GSK, at the 2021 Veeva R&D and Quality Summit Connect.
Here’s how GSK is modernizing the way it approaches clinical data management—an undertaking that’s involved replacing multiple EDCs and complex processes—and how it made progress in half the time expected, accelerating delivery in 18 months due to an agile methodology.
Putting data at the center
Historically, explained Mayank, the industry had overlooked clinical data for years, preferring to focus on medical writing, stats, and submissions systems. But now, as companies embrace hybrid digital trial models and struggle with a proliferation of digital data sources, effective data management has become a key driver of trial success.
That’s why GSK decided to implement Veeva CDB, a clinical database that speeds data cleaning and standardization to allow its data to be managed, reported, and analyzed. Not only will this make clinical research more agile, but it will also enable more advanced tools, such as NLP and visualizations, to be applied, while making artificial intelligence (AI) and machine learning initiatives more successful in the future, Mayank said.
Identifying GSK’s “why”
As the company began its modernization project, it started by clearly articulating its “why” for change. As Mayank noted, GSK’s three main goals were to:
- Simplify the operating model. Before adopting Veeva, GSK was using at least four different types of EDCs within its clinical data ecosystem, and each of them had been customized to such an extent that the result resembled a jigsaw puzzle. It was important to reduce the complexity that had built up over the years.
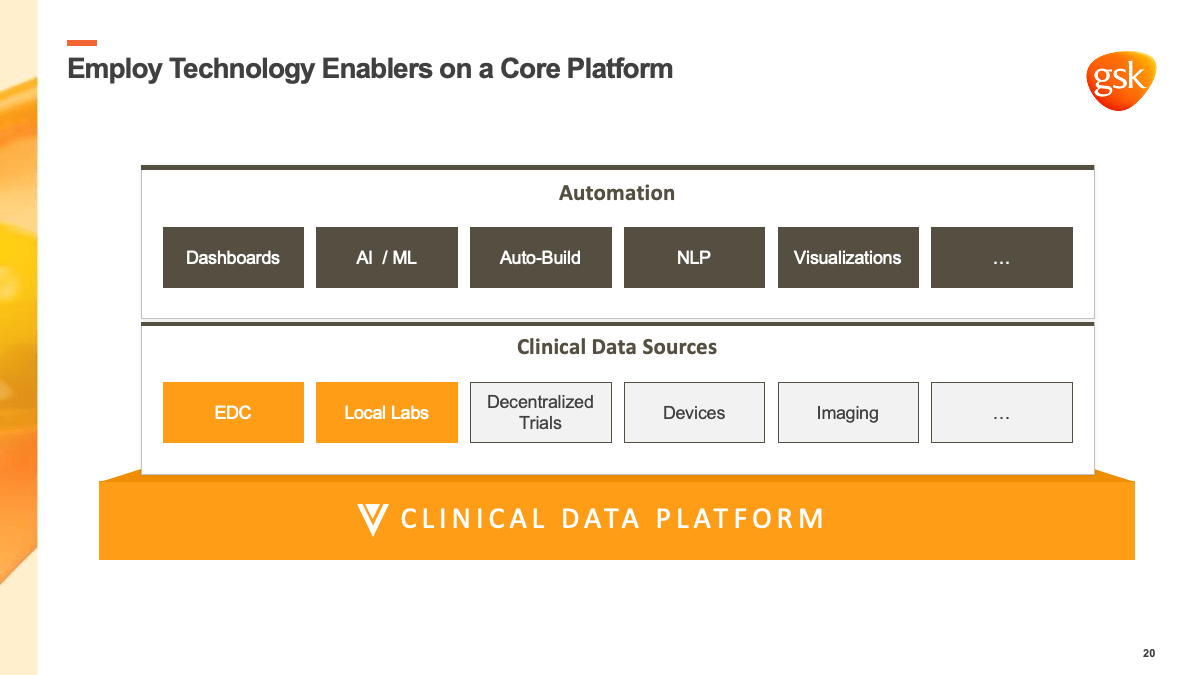
- Employ technology enablers. As Mayank explained, the industry interpreted technology very narrowly, as though changing the EDC platform was enough. However, there are many different technology enablers to help clinical teams do their jobs better, with significant advances in areas such as AI and machine learning to leverage.
- Accelerate delivery. Mayank emphasized the importance of speeding up the delivery of life-saving treatments with a sobering statistic: for certain diseases, two afflicted patients die every minute. “Our patients are waiting,” he stressed. “Just imagine the number of lives being lost if the industry continues to do clinical study locks in 21 weeks, instead of four or even two weeks.”
Aligning on a single CDM platform
To escape the complexity of its jigsaw puzzle of EDCs, GSK adopted Veeva Vault CDMS as its single, core CDM platform. However, this time, its data specialists avoided customization and, instead, chose a platform based on its own inherent capabilities. “Once you adopt a new platform, you shouldn’t spend the next 10 years recreating the same complexity that you had with the previous one,” Mayank said.
Moving to a single platform will ultimately help GSK improve data review. Over the years, the company had created additional complexity and slowed its processes by collecting and reviewing every single data point from a trial. It treated all data as critical, when in fact, “all data is not equal,” said Mayank, explaining that value-driven data cleaning is now a key strategy.
Veeva CDB will aggregate and harmonize clinical data from every source (EDC, imaging, eSource, ePRO, local labs, and more) to centralize cleaning and reconciliation. Meanwhile, technology enablers will support data cleaning and exploring to surface trial insights. As data managers evolve to become data scientists, these enablers will allow them to be more effective in their roles, helping consolidate all data into one place for faster time to insights.
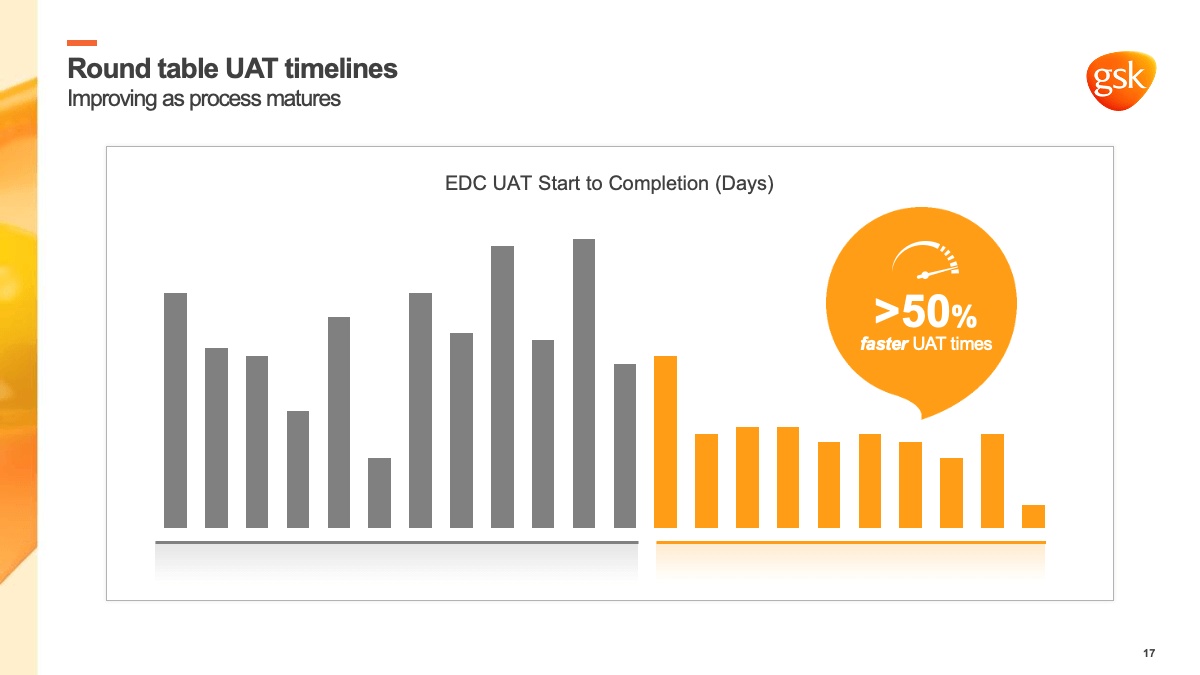
Setting a new benchmark
Accelerating delivery, however, requires more than a simplified operation model and alignment on a single platform—it requires a mindset change as well. “The question on many people’s minds was, ‘If we can deliver on accelerated treatments for COVID, why can’t we do it for everything else? What is stopping us?’” said Mayank.
GSK was already meeting the industry benchmark for delivery, but that wasn’t enough. “We want to do at least 50 to 60% better than what the industry is achieving, and not just for selective studies, but across the portfolio,” said Mayank. “That’s what we are striving for with Veeva, to become the industry benchmark for the future.”
The importance of agile development
Mayank stressed that partnerships with Veeva and other technology providers have been key to rapid transformation. “We were on this journey together,” he said. As a result, the team completed the project in just 18 months, largely due to the agile development process that the teams used for collaboration.
Agile has become something of a buzzword, Mayank acknowledged, with every organization wanting to get on the bandwagon. However, taking that approach had a significant impact on GSK’s ability to move faster and accelerate delivery. Agile, he explained, “is helping us to fail faster rather than failing later, and that saves us a lot of time as we progress.”
GSK’s modernization journey is not over, and the company plans to continue collaborating with Veeva and other partners to continually improve. “Every year, we want people to stand up and challenge what we are doing. I think that’s the only way to become the benchmark for the industry,” Mayank said. “Ultimately, this will benefit our patients, who will get their drugs and vaccines much faster.”
Watch the presentation to learn how Vault CDMS is giving GSK a modern foundation to accelerate their future goals.