Blog
GSK’s Journey to Accelerated Study Delivery
Sep 23, 2020 | Ashley Davidson
Sep 23, 2020 | Ashley Davidson
In a recent webinar, Jess Robinson, Director of Study Start-up at GSK, highlighted the importance of aligning people, processes, and technology when embarking on a study start-up transformation journey.
A deep dive into cycle time metrics for key start-up milestones was the catalyst for change at GSK. After benchmarking their performance against industry standards, they realized they needed to change how they work to optimize clinical delivery.
GSK understood implementing new technology in a silo without considering the impact on people and processes would not be successful, so they incorporated all three pillars into their Vault Clinical deployment to work smarter, better, and faster.
GSK understood implementing new technology in a silo without considering the impact on people and processes would not be successful, so they incorporated all three pillars into their Vault Clinical deployment to work smarter, better, and faster.
PROCESS
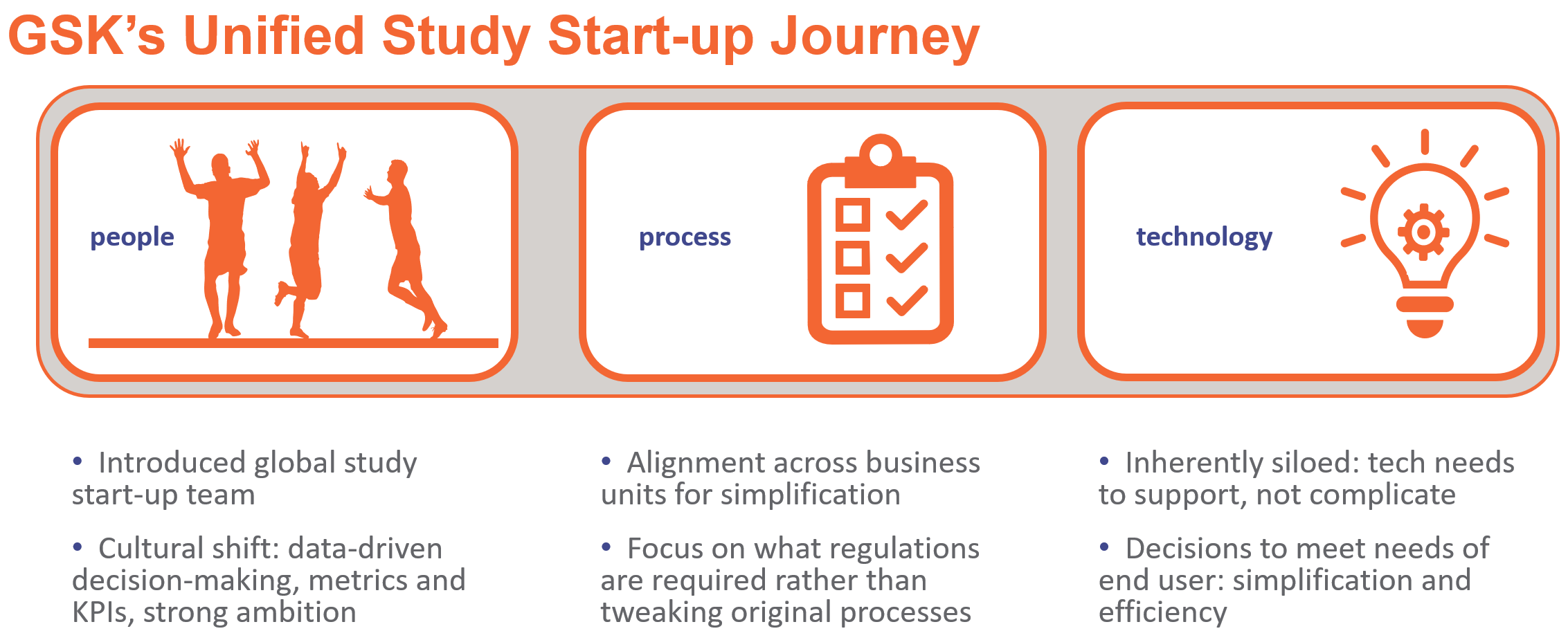
Rather than adjusting old processes to fit a new system, GSK’s global study start-up team partnered with business owners to re-engineer processes to fit the new ways of working. With a focus on improving efficiencies through simplification, the technology and business teams collaborated to establish project objectives, define goals, and gain alignment.
Rather than adjusting old processes to fit a new system, GSK’s global study start-up team partnered with business owners to re-engineer processes to fit the new ways of working. With a focus on improving efficiencies through simplification, the technology and business teams collaborated to establish project objectives, define goals, and gain alignment.
PEOPLE
Because a transformation project requires significant internal resource investment, GSK developed strategies that mapped to key activities such as engagement with stakeholders, and training and communication plans to ensure business readiness. They identified change champions throughout the organization to evangelize the new workstreams, drive accountability across business owners, and assist study country teams with the technology rollout.
Because a transformation project requires significant internal resource investment, GSK developed strategies that mapped to key activities such as engagement with stakeholders, and training and communication plans to ensure business readiness. They identified change champions throughout the organization to evangelize the new workstreams, drive accountability across business owners, and assist study country teams with the technology rollout.
TECHNOLOGY
Eliminating siloed workflows, transitioning to data-driven decision-making, and simplifying the experience to improve productivity for end-users and sites were key drivers for GSK that shaped the decision criteria for the new technology. A streamlined user interface, on-screen navigation cues, and visibility to KPIs and metrics for different functional areas greatly improved technology adoption.
Eliminating siloed workflows, transitioning to data-driven decision-making, and simplifying the experience to improve productivity for end-users and sites were key drivers for GSK that shaped the decision criteria for the new technology. A streamlined user interface, on-screen navigation cues, and visibility to KPIs and metrics for different functional areas greatly improved technology adoption.
Fostering change across the people, process, and technology pillars has had a meaningful impact on GSK’s operations and ability to accelerate study delivery. Findings from the Study Start-up Pulse Report also show that modernizing study start-up systems and processes is one of the greatest areas of opportunity to improve trial performance and bring treatments to market faster.
Stay tuned for the next blog in the series, where we’ll explore unified vs. integrated study start-up technology and the value for GSK in more detail.