Blog
Quality Management Across Your Global Supply Chain
Apr 20, 2017 | Sandra K.Rodriguez
Apr 20, 2017 | Sandra K.Rodriguez
Taking Back Control of Quality
Globalization and outsourcing has created unique opportunities and demanding challenges for the industry and regulators alike. Requirements and standards are also evolving, and legacy solutions are incapable of meeting new business needs. These trends call for a new paradigm in global supply chain management.
Historically, data was managed by the brand owner – close by and easily monitored. With outsourcing, quality oversight is strained at best. To take back control, companies must have access to quality metrics in real-time. This approach offers three key benefits:
Improving Supply Chain Visibility, Control and Collaboration
Growth in foreign and outsourced facilities as well as increased variety and complexity of globally sourced products is forcing companies to rethink three core aspects of supply chain: visibility, control, and collaboration.
-
Visibility – With improved visibility, companies can better manage risk and make more informed decisions. Systems that provide access to internal groups as well as external partners can increase alignment and strengthen partnerships.
-
Control – Providing management access to critical supplier data increases control over the supply chain and the ability to comply with track and trace requirements.
-
Collaboration – Finally, by collaborating with suppliers and regulators, organizations are better positioned to adhere to the changing regulatory landscape and continually improve quality.
Without proper visibility, control, and collaboration, brand owners run the risk of not only poor product quality but also regulatory backlash for failure to comply with existing regulations.
Is Your Quality Control Unit (QCU) Ready for Metrics?
Quality metrics are used throughout the drug and biologic industry to monitor quality control systems and processes, and drive continuous improvement efforts in manufacturing.
The FDA Quality Metrics Program intends to calculate the following based on industry reporting:
The agency asserts that the ‘product reporting establishment’ has a responsibility for oversight and controls over the manufacturing of its drugs to ensure product quality. In addition, they state the QCU at ‘product reporting establishments’ should already possess, or have access to all of the quality metrics data needed for the report.
Not More Systems, Just Better Systems
FDA has increasingly observed cGMP violations involving data integrity issues during inspections. Ensuring data integrity is an important component of ensuring the safety, efficacy, and quality of drugs. To address data integrity risks and violations, the answer is not necessarily implementing more systems.
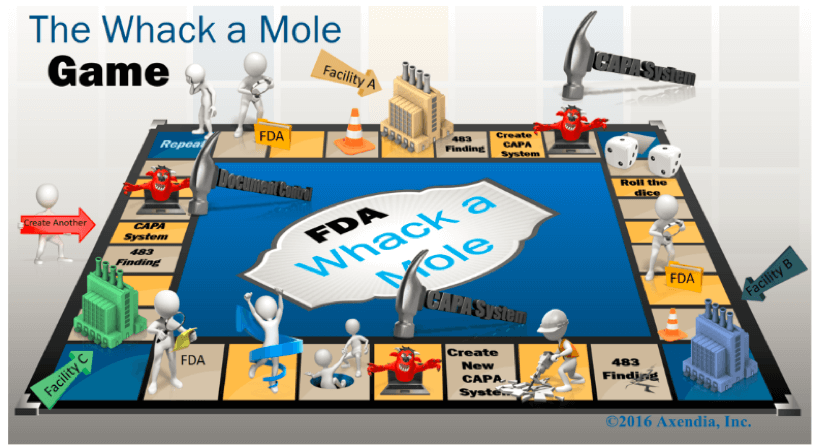
In fact, the industry already suffers from a multitude of stand-alone systems. Functional areas have historically implemented a system to address a specific business need or respond to a regulatory action – leading the industry to become experts in playing regulatory whack-a-mole.
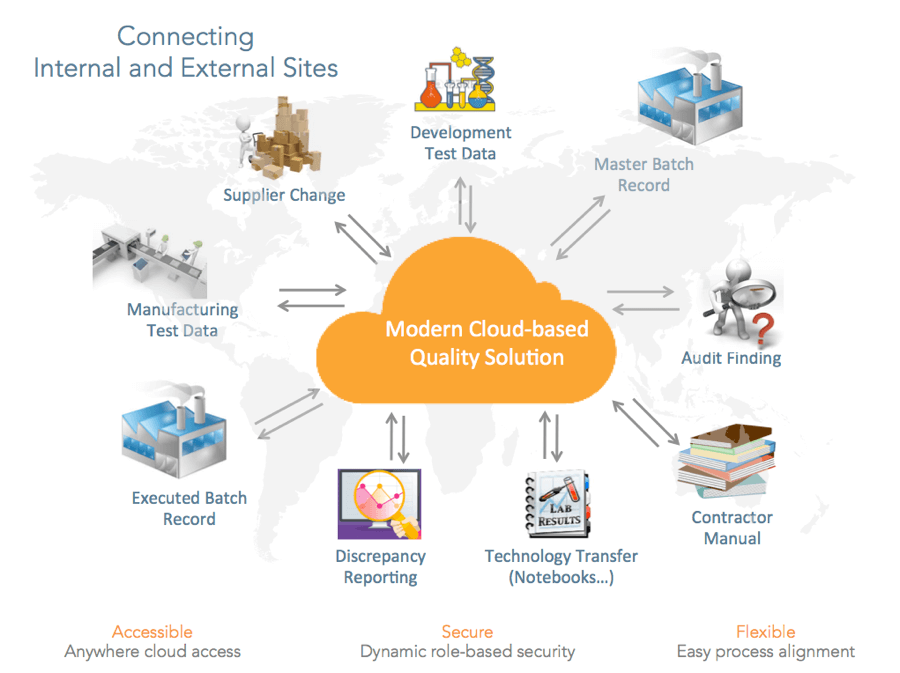
For example, a 483 observation at one company’s facility prompts a system implementation to address the issue – whacking one mole. The FDA then goes to the next facility issuing another 483. The company responds by implementing a different system – whacking another mole – instead of looking at the problem holistically. Many legacy systems are siloed, preventing a broader understanding of how quality events are related. Modern cloud-based quality systems make it easy to bring together geographically dispersed groups and disparate data to gain a more complete picture of quality.
Adopting Smart-Sourcing Strategies
Brand owners should adopt “smart-sourcing” strategies; evaluating the total cost and potential risks, not just the initial investment. By implementing a commercial, legal, and technological framework that promotes the exchange of information, external partners can become a true extension of the brand owner’s quality and information system.
Functional areas were traditionally vertically integrated. With outsourcing, brand owners no longer generate, or own the data, within their four walls. Third parties – that are often located in a different part of the world – generate the data. This makes it difficult to collect the data and gather intelligence.
Assimilating quality metrics data for submission to the FDA can be tricky in an outsourced environment. Brand owners often generate data from quality management (QMS) and complaint handling systems, whereas manufacturing data from an enterprise resource planning (ERP) system or other quality event information reside at a supplier, contract manufacturer (CMO), or other partner.
In order to modernize quality management, brand owners should consider:
Modernizing with Cloud
Modern quality management incorporates QMS processes and quality-related content in one unified system. This allows users to do their work more efficiently and provide complete end-to-end visibility over processes that span documents and data or across functional areas, such as change management or change control.
Cloud-based QMS systems can help drive global transparency with a single source of truth for quality-related data and processes. Cloud solutions offer easy, online access anywhere in the world. However, dynamic, role-based security features must be built-in to the solution and work flows need to easily align with business processes. With solutions such as Vault Quality Docs and Vault QMS, brand owners have access and full control over their data regardless of where it is generated.
Armed with data and strengthened by intelligence from modern QMS solutions, companies can move from reactive to predictive quality organizations.
Reactive – Management often focuses on issue and task tracking, investigating issues as they occur.
Proactive – Management has access to dashboards with key performance indicators (KPIs) to monitor supply chain related activities and make adjustments.
Predictive – With visibility into the entire supply chain, management can identify trends and focus on continuous process and quality improvements that unify internal and external quality.
Regulators are encouraging industry to leverage modern quality management systems to improve visibility, control and collaboration across the supply chain. To hear more, the webinar is available on demand here.