Blog
Rewriting the Business Case for Regulatory Transformation
Feb 29, 2024 | Kim Brownrigg
Feb 29, 2024 | Kim Brownrigg
Regulatory teams making the case for digital transformation often encounter the same obstacle: they must persuade leadership to finance a long-term investment without visibility into potential regulatory changes, technology advancements, or company strategy.
As Bayer and another top 20 biopharma company explain, a foundation in technology and data is a powerful enabler in a dynamic environment. But it is also just the beginning of the journey. To secure support for ongoing regulatory transformation, your business case needs to demonstrate both the immediate and long-term value that will be realized after implementation.
Traditional dimensions won’t work
Regulatory transformation programs are often multi-year processes. As a result, it can take some time to build a robust business case, not least because fast-moving targets, a changing technology landscape, and a dense regulatory agenda are making it harder for biopharma companies to see around the corner.
A successful business case will align with company strategy and answer the question: how will this investment support our broader mission? The aspiration could be to respond more nimbly to regulatory requirements, for example; or ensure more efficient ways of working across a global footprint. As one global head of regulatory operations at a large biopharma company notes, “The world is spinning faster every year… And focusing only on the traditional dimensions of efficiency, acceleration, and compliance won’t give you a compelling business case.”
In our experience, the most effective teams anticipate that their investment must show value almost immediately and establish which key performance indicators (KPIs) will consistently provide the required data. Mark Davies Karvonen, global product manager at Bayer, notes: “We get asked about the value delivered by the program almost every week. We would like to prove it with data.”
Once the business objectives have been agreed upon, each of these can be mapped to associated benefits, value drivers, and KPIs that will be realized over time [Figure 1]. By defining how to realize value in your business case, you can start collecting data soon after signing off.
Figure 1: Defining the path to value realization
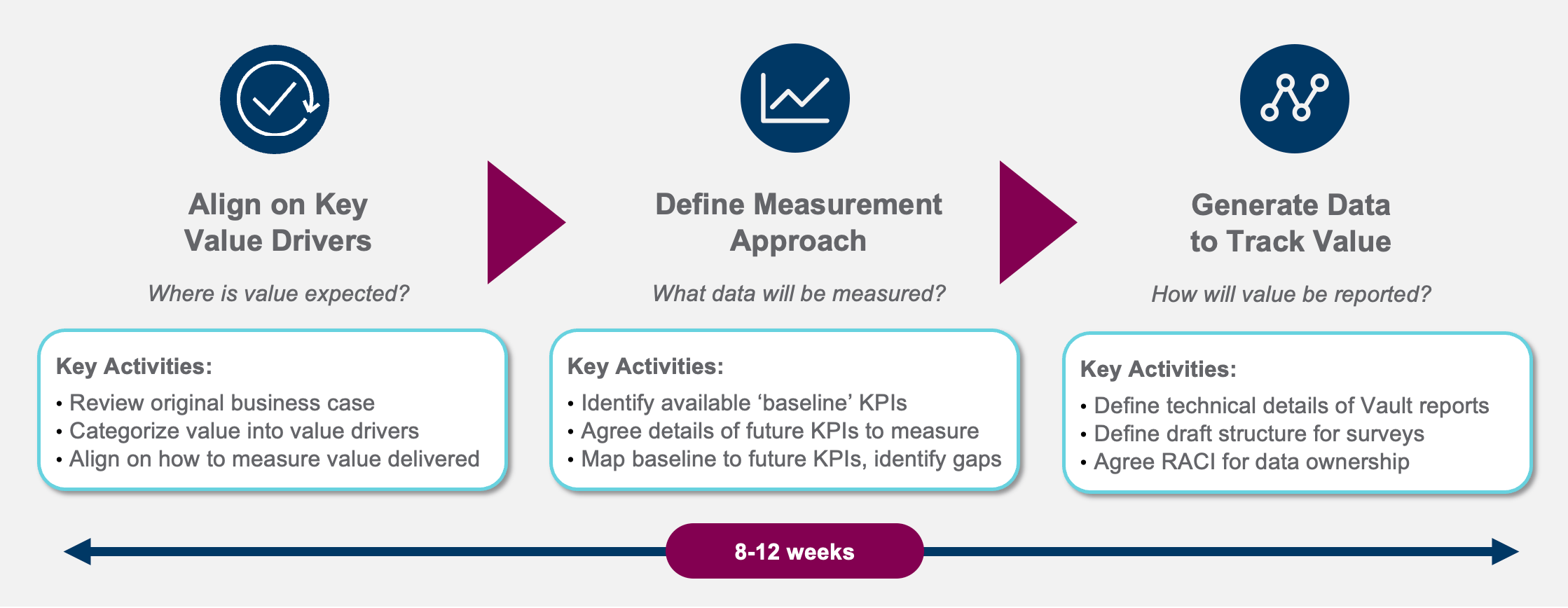
Source: Veeva Business Consulting
Collecting data that counts
It’s easier to start measuring once the wider business agrees on where it expects to realize the most value. Quantifiable KPIs should flow from the benefits and value drivers you have defined [Figure 2].
Figure 2: KPIs should flow from value drivers and benefits
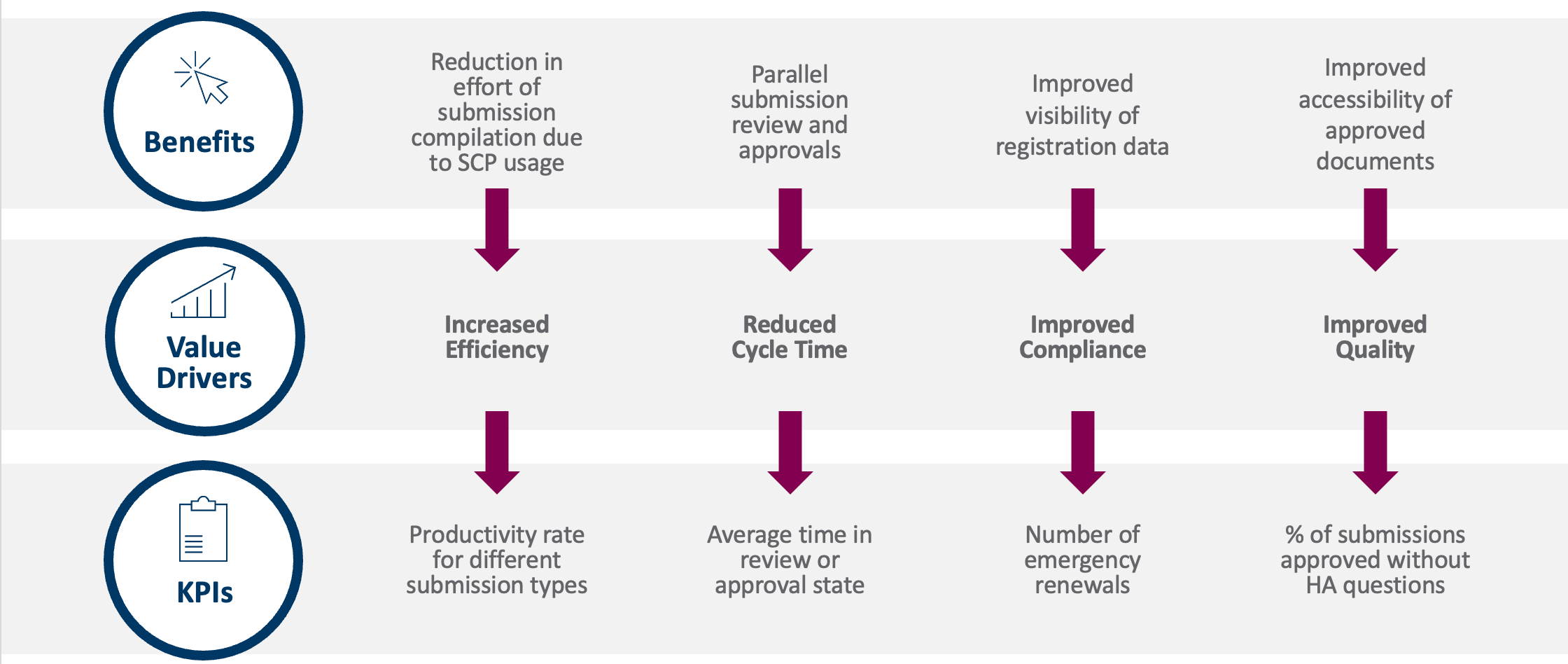
For instance, if an intended benefit of implementation is to undertake parallel submission reviews and approvals, your value driver is reduced cycle time. A relevant KPI could then be the average time that a document sits in review or approval. Alternatively, your value driver might be improved quality (e.g., so that your business has better access to approved documents to build submissions), in which case the data required could be the percentage of submissions approved without health authority questions.
While it may be tempting to try and measure every possible improvement, a focused approach pays off. The most effective way to build stakeholder buy-in and ensure good governance is to have an executive-level sponsor for each KPI, which means only having as many as necessary. Involve subject matter experts to select between 10 and 15 KPIs that can be tracked over time. Once these have been confirmed, clarify roles and responsibilities within your team for collecting and reporting data.
Baseline KPIs are an important part of the measurement approach. Occasionally, baseline metrics can be sourced from data captured in legacy systems before implementation. If this isn’t possible, then a snapshot six months into the program may work as a substitute baseline. Some of these KPIs will later change, but that shouldn’t put you off. As Davies Karvonen notes, “Value realization means using the KPIs you’ve created.”
Telling your story to the business
A critical component of stakeholder management is communicating the benefits of your transformation program. Teams that take a 360-degree view of their stakeholders are better placed to select the most persuasive arguments. Knowing who the decision makers and influencers are in the process means you can step into their shoes to determine the benefits that matter most to them. Each group will have its lens, and your storytelling has to address their perspectives to win hearts and minds.
Less is more when telling your story to the business. The global head of regulatory operations emphasizes, “Business cases that are 50-80 slides never go anywhere. If you need 80 slides, you don’t have a good story to tell. Only include your strongest arguments, and it should take no more than 10 slides.”
Keeping the wider audience close and informed also generates dividends in the long run. When stakeholders lack a good understanding of the business case, their default response is often ‘no’. They advise, “Memories are short, and new people will join while others will leave. Maintain a record of what you’re doing and keep repeating the key messages from your story.”
Finally, leading companies don’t wait to take action as data starts coming through. For instance, if one area of your business is moving slower than expected, it’s worth adapting resourcing across functions. This strengthens the value realization story once the new platform is live.
Embracing ‘transformation as usual’
Developing a robust business case for a multiyear process is challenging, but it is always reinforced by a systematic approach to collecting data and measuring value. Good data will address stakeholder questions, and serve as a continual reminder of the tangible business value delivered and how transformation supports the company’s mission.
The global head of regulatory operations concludes: “We learned that siloed systems were shaping our activities. As we implement Veeva, we have ‘aha’ moments as the teams realize that we can change our dynamic, footprint, and ways of working.”Register to attend Veeva R&D and Quality Summit in Madrid to learn about regulatory transformation.