Blog
Showcasing Mid-study Updates in Vault CDMS at Summit Connect
Jun 07, 2021 | Natalie Townsend
Jun 07, 2021 | Natalie Townsend
Despite being a virtual event, the Summit participants attending my demo were actively engaged and had pressing questions about the study build capabilities in Vault CDMS. We covered a range of topics including, property-based configurations, eliminating custom functions, and using a library with true lineage reporting. However the bulk of the questions were around Veeva’s migration-free mid-study amendments.
The audience was keen to understand how they worked, so I used the four following questions to guide them through Vault EDC’s migration-free approach to mid-study updates.
1 – How easy is it to make changes?
The modern build tools in Vault Studio make it simple to make changes. And there are error checking tools that help prevent making changes that may be detrimental to the current design or existing data. In the demonstration, I added a new ECG form to the screening visit, added fields to the ICF and vital signs forms, and showed how quickly you can reorder forms and fields. All this is achieved via the intuitive schedule editor view and drag and drop form design. More complex amendments can be managed too and Veeva have best practice advice on handling a full range of different scenarios.
2 – How do I know what has changed?
Using the difference report, users can see what has changed between versions of the casebook, sense check that nothing was changed inadvertently, and focus their validation efforts. It’s also powerful when comparing a study build to a library collection to identify where teams have reused, deviated from, or added to standards.
3 – How do I roll out the changes?
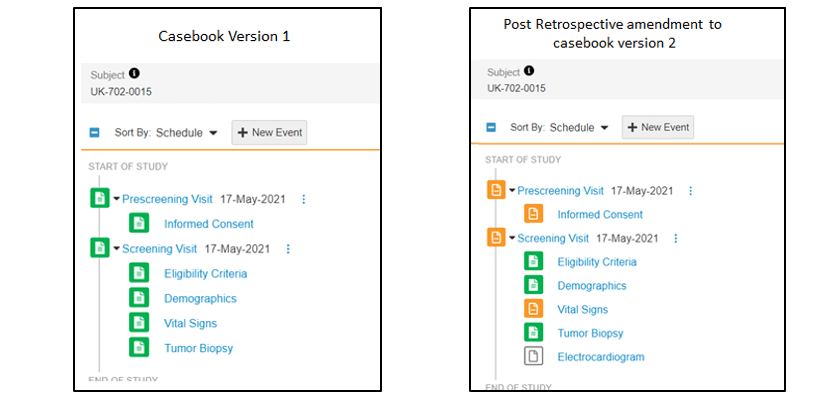
When making changes to a casebook definition, such as adding new forms and fields, Vault creates a new version of the casebook. These different versions can coexist across sites and even within a site. In the live demonstration, I moved a site from casebook version one to version two, meaning that all new patients at that site would instantly have version two applied. I also applied this to an existing individual patient retrospectively, demonstrating the speed and reliability of amendments in Vault CDMS. As there are no obstacles such as data migrations or downtime, in just a few clicks these changes were applied and visible to the audience. Comprehensive reporting is available across the study to track which patients have what events on which casebook version.
4 – What does this look like for site personnel?
I am commonly asked about the site experience when existing patient data is affected by an amendment. Vault CDMS clearly highlights where personnel need to take action as the result of an update.
In the demonstration example, the patient changed from having a completed prescreening and screening event (Figure 1. below), to requiring new data on their informed consent and vital signs forms, as well as having a completely new ECG form to complete (Figure 2 below).
The color-coded iconography in the data entry screen makes it clear that there are new actions required. The screenshots below show the visit and form icons changing from green, which signified that everything was complete, to orange, which signifies additional data collection is required.
There are many innovative features that could be discussed for CDMS study builds, but it’s clear that managing mid-study amendments effectively and efficiently remains a challenge, and one that Vault CDMS advanced study build capabilities allows sponsors and CROs to overcome.
For further information:
Implementing Study Amendments Without Downtime or Data Migrations