Blog
The 411 on Efficient Monitoring in Vault CTMS
Nov 06, 2019 | Kyle Stephenson
Nov 06, 2019 | Kyle Stephenson
Because monitoring accounts for 25-30% of overall trial costs,1 life sciences companies and contract research organizations (CROs) are always looking for ways to optimize their monitoring processes. In this post, we’ll cover how the use of monitoring reviewer comments enables more efficient monitoring in Vault CTMS. Then we’ll get the real-world perspective from Ora, a full-service research organization specializing in ophthalmology, in our next blog.
What is a Monitoring Reviewer Comment in Vault CTMS?
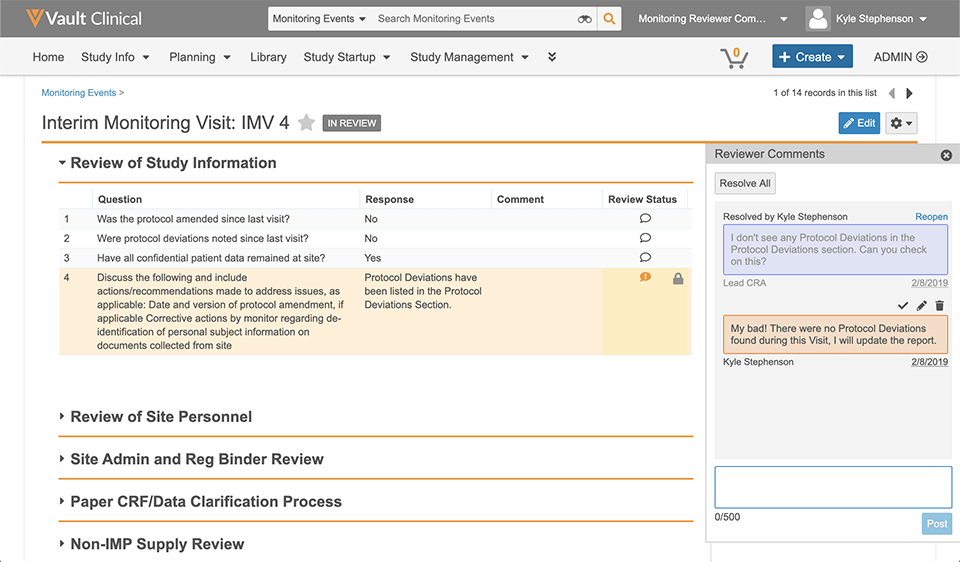
Reviewer comments allow CRAs and lead CRAs to quickly provide feedback on monitoring events directly in line with monitoring event data. They can post comments, respond, and resolve them in Vault CTMS. All comments, both open and resolved, can be tracked in the review summary section on the monitoring event.
The Basics of Monitoring Reviewer Comments
Let’s review a common scenario to illustrate the power of monitoring reviewer comments in Vault CTMS.
CRA Experience
- Krista CRA logs into Vault CTMS and reviews the CRA homepage to get an at-a-glance view of site summary metrics, planned monitoring events, and tasks.
- Krista drills into a planned monitoring event to start her trip report. Subjects and subject visit SDV syncs from Vault EDC to Vault CTMS. Other study information, such as trip report questions and answers, protocol deviations, and applicable monitoring follow-up items, are also included in the report.
- All looks good to Krista, so she submits the monitoring event to Lucy, the lead CRA, for review.
Lead CRA Experience
- While reviewing the monitoring event information, Lucy sees a note that a protocol deviation was logged because the wrong version of the ICF was signed by a subject. When reviewing the protocol deviations associated with the monitoring event, she notices there isn’t an actual record of the protocol deviation.
- Lucy adds a monitoring reviewer comment directly in line with the original note so Krista knows she needs to log the deviation.
- Lucy sends the event back to Krista, who can see a summary of all review comments and their statuses.
- Once Krista corrects the issue by logging a protocol deviation, Krista and Lucy can both eSign the monitoring visit (ICH E6 R2 compliant!), which creates a trip report that’s automatically finalized and filed in Vault eTMF.
How Data-Based Monitoring Processes Increase Efficiency
Inline monitoring reviewer comments are just one way that Vault CTMS enables data-driven monitoring. Here are five other ways Vault CTMS leverages data to improve monitoring:
- Data on monitoring events are automatically populated on trip reports, increasing efficiency and study quality.
Follow-up items, monitoring activities, subjects, subject visits, and enrollment metrics are populated automatically, reducing data entry errors and enabling more efficient processes. - Real-time data validation ensures all relevant fields are populated and reviewer comments are resolved before a monitoring event is finalized.
Automation and built-in error-checking improves data quality and significantly increases productivity since lead CRAs no longer must validate the granular details of the trip report. - Subject and subject visit data from Vault EDC is automatically available in Vault CTMS.
Subject and subject visit information from Vault EDC automatically syncs to Vault CTMS, removing manual effort, minimizing data entry errors, and saving time. - Inline reviewer comments eliminate emails that can’t be tracked and audited.
CRAs can review comments from lead CRAs and study managers directly within the application where the data was entered. They can make corrections to the monitoring event, the same place they do their work. - Monitoring events automatically generate trip reports in Vault eTMF.
As part of a unified clinical operating environment, documents generated in Vault CTMS are automatically populated in Vault eTMF, without requiring complex integrations or manual processes.
Check out our next blog where Ora shares their experience and outcomes with monitoring reviewer comments.