Blog
What Can You Automate with Veeva’s EDC to CTMS Connection?
Jan 31, 2023 | Karen Klein and Drew Garty
Jan 31, 2023 | Karen Klein and Drew Garty
Automation is powerful. Data from one system flows automatically into another, surfacing where you need it, and completing tasks that were once manual. The problem is that automating tasks between systems typically requires integrations, and integrations are fraught with challenges. Most are highly complex, which makes them fragile and susceptible to breaking. Integrations can also impact system scalability and increase the risk of compliance breaches. This technical complexity makes managing integrations difficult for IT, and drives the lengthy and expensive setup times.
Cross-system automation also requires data mappings, which are themselves often complicated and error prone. When data transfers from your EDC to your CTMS are missing or inaccurate due to mapping errors or shortcomings, clinical teams spend hours identifying, investigating, and correcting the problems.
The Vault Clinical Operations to EDC Connection removes these fragile, point-to-point integrations and simplifies the bi-directional movement of data between your EDC and the Vault Clinical Operations suite. The productized connection is designed for accuracy, durability, and performance in a highly transactional environment.
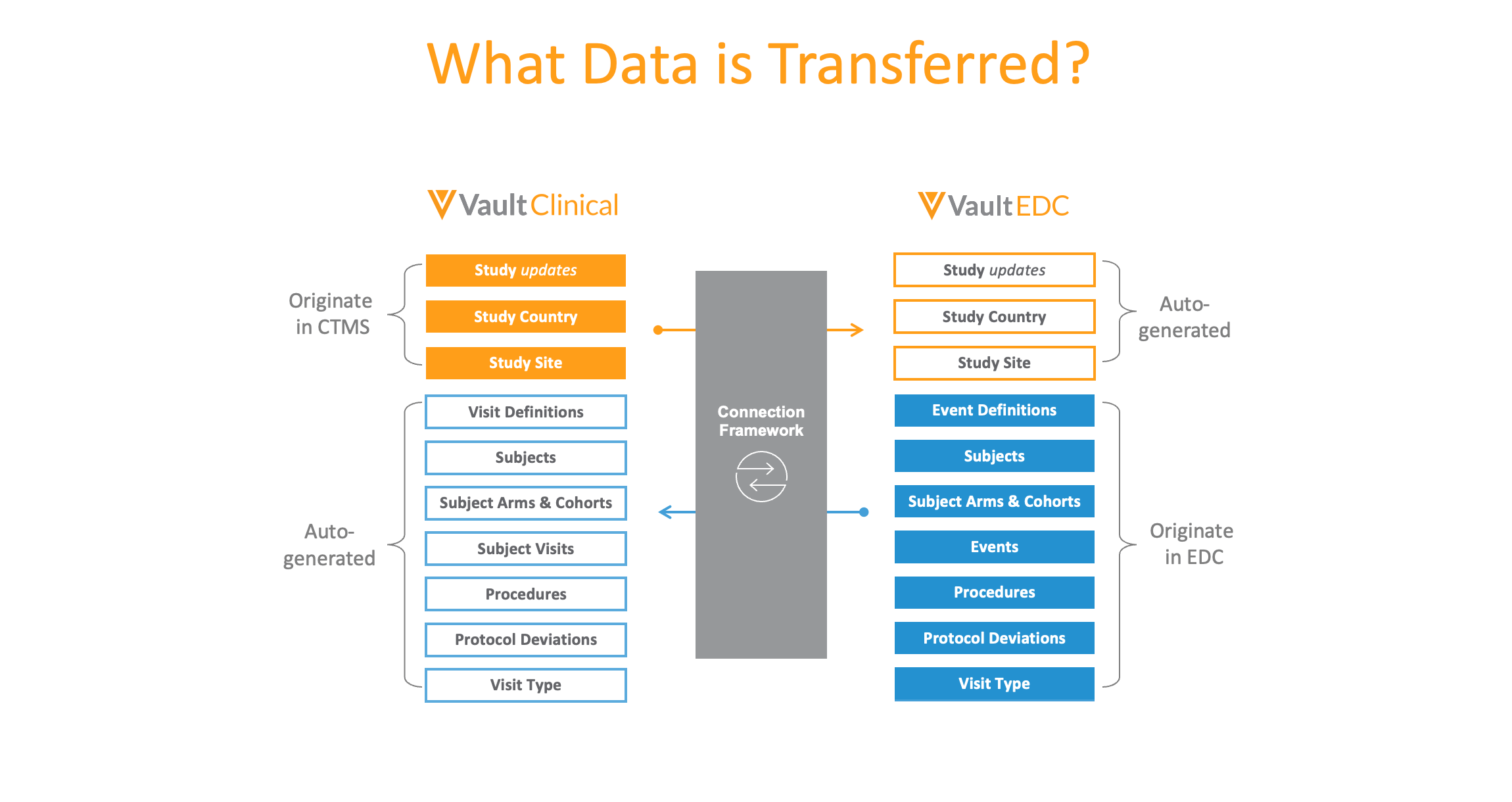
Study-level data, including study country and study sites, automatically move from Vault CTMS to Vault EDC; while subject and visit-level data, along with procedures and protocol deviations, move from the EDC to CTMS, eliminating the need for duplicate data entry. (Technically, the data moves between Vault EDC and the Vault Clinical Operations suite, where all the applications, including Vault CTMS and Vault Payments share data.) The connection spares data managers and CRAs from running reports, exporting data, or performing reconciliations on behalf of their colleagues. Each team works within their own applications, reducing the number of systems they need to touch.
Since Vault Connections can trigger actions in addition to transferring data, we think about their capabilities from a business process perspective. The Vault Clinical Operations to EDC Connection currently automates aspects of five distinct processes: EDC setup, CTMS reporting, deviation management, site monitoring, and payments. The key capabilities and benefits include:
- Populate study and site-level data into Vault EDC. This minimizes duplicate data entry and ensures data consistency, which is critical to successful reporting.
- Populate subject and visit-level data into the Vault Clinical Operations suite. This keeps CTMS reports current with the latest enrollment and subject milestone information, supporting timely metrics and informed decisions.
- Transfer protocol deviations identified in Vault EDC to the issue management hub in Vault CTMS. CRAs get better visibility into issues and spend less time entering and reconciling data.
- Automate manual monitoring tasks from planning through reporting by creating monitored subjects and visits, seeding SDV status and deviations in monitoring events, and populating trip reports with subjects, visits, SDV completion, and more.
- Automatically calculate and initiate payments in Vault Payments based on visit and procedure data from Vault EDC. Sites get paid faster, which strengthens your site relationships.
As you can see, study execution data is available when you need it, where you need it. Having a deep and reliable connection between your EDC and CTMS helps you manage trials more efficiently. The automation not only completes tasks faster, it enables you to shift your focus from ensuring data quality to making good decisions–and acting on them.