Blog
The Three Pillars of Study Oversight to Drive Trial Success
Aug 08, 2023 | Chris McSpiritt
Aug 08, 2023 | Chris McSpiritt
Study oversight is a crucial component of study conduct, yet it’s often a hotly debated topic among biopharma companies that outsource study management to contract research organizations (CROs). Many are unsure what regulators want to see and what they should do to comply. On a recent webinar, 57% of attendees didn’t have a good understanding of what’s required to show proper oversight. When asked what oversight means, sponsor responses varied widely:
- We maintain a log of meeting minutes and review dashboards
- We focus on metrics, monitoring, and critical risks to ensure inspection readiness
- We want our CRO partners to be fully transparent and have active discussions with us so we’re aware of issues like protocol deviations
In addition to lack of clarity on what study oversight actually entails, other common challenges I hear include:
- Collaboration. Sponsors want more effective upfront collaboration with their CRO partners to understand what KPIs and data they can provide to help them demonstrate oversight.
- Access. Oversight becomes incredibly difficult and there is a lack of trust and transparency when sponsors are unable to run self-service reports and must rely on weekly exports from their CROs.
- Action. Even if sponsors have a detailed oversight plan jointly approved with their CROs, they encounter obstacles driving action, especially if CRO employee attrition is high. There is a noticeable knowledge gap between monitors, especially with complex studies.
This data clearly highlights there is industry-wide uncertainty. My goal in this blog is to provide a clear definition of oversight.
What is oversight?
GCP guidelines state that sponsors are accountable for trial conduct, even when trials are outsourced to CROs.
“A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a CRO, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor.” ICH E6 (R2) Addendum
Documentation as evidence of CRO and trial oversight is just one small piece of the puzzle. At Veeva, we believe there are three core pillars to achieve effective study oversight:
- Manage and understand your portfolio
- Oversee CROs and vendors
- Comply with regulations
Let’s take a deeper look at each pillar.
Manage and understand your portfolio
Sponsors should actively monitor and manage overall study performance – site enrollment, site performance trends, and issues that impact timelines – across all CROs and be able to answer these questions at any time during the study:
- How are we tracking to planned dates?
- How are we tracking for site activation per country?
- Are we on track with enrollment?
- Which sites are low-to-no enrolling?
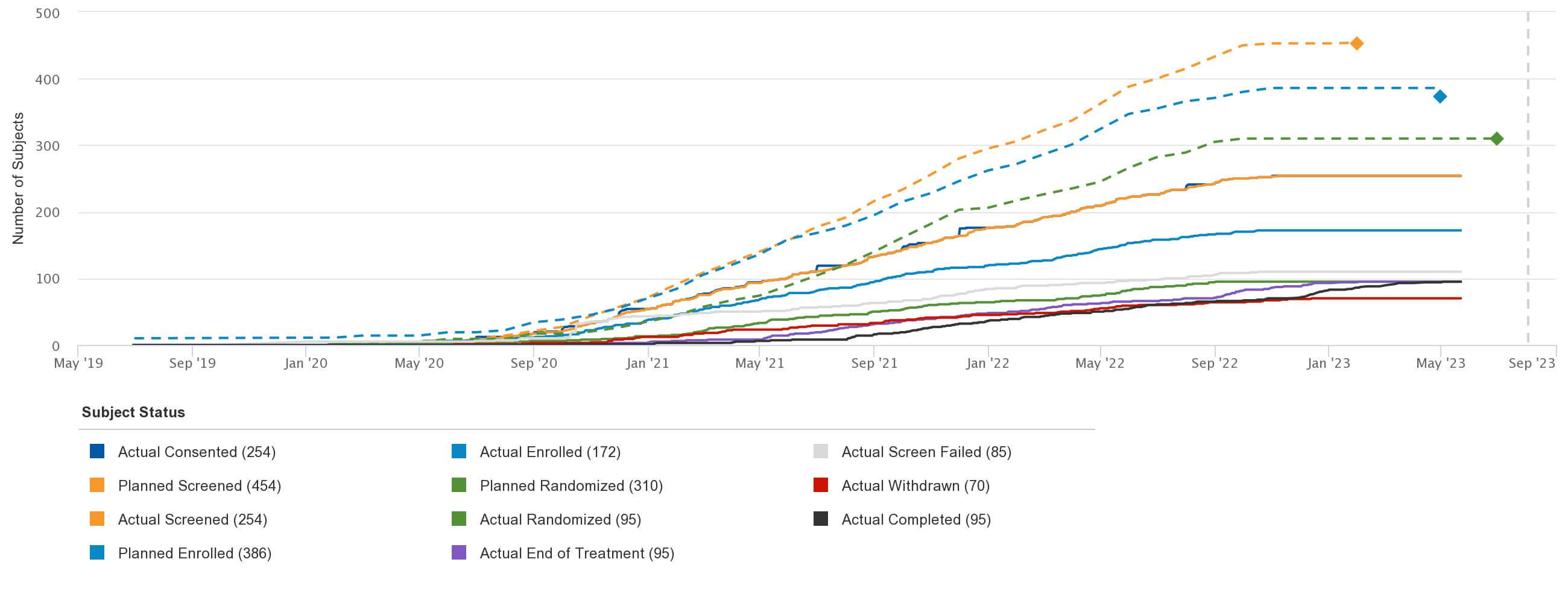
Figure 1: Study Enrollment Status
Easy access to this level of information across CROs drives a better understanding of historical site performance and informs future site selection. Sponsors should know if these sites and countries can reliably be used in future trials.
Additionally, sponsors can reduce issues that may impact study timelines and outcomes through proactive risk identification. Sharing this information with leadership early, as well as documenting issues and corrective actions, is a great way to establish a stronger rapport.
Oversee CROs and vendors
Ensuring your CRO partners have delivered to your expectations is another important aspect of study oversight. Sponsors should understand if CROs are tracking to expected service-level agreements (SLAs) and engage in collaborative discussions to align on metrics. Common metrics sponsors should measure are:
- Time to query resolution
- Count of outstanding queries
- Recruitment plan vs. actual
- MVR quality findings
- Issue management, resolution, and issue trends analysis

Figure 2: Issue Types by CRO
For example, visibility to the number of open queries and remaining source data verification (SDV) would be very helpful at the end of a study when you’re pushing to close out sites.
Having discussions based on data also provides quantifiable figures as you approach contract negotiations for future studies. Having this data helps eliminate the emotions and focus the discussion on facts.
Comply with regulations
The last pillar of study oversight spotlights the regulatory component and the key activities sponsors can do:
- Establishing a risk management strategy with CROs per study
- Ensuring the study team roster has been reviewed before providing it to an auditor
- Triaging observations/issues and having a closed-loop system to ensure they are adequately addressed
- Conducting co-monitoring visits and reviewing monitoring visit reports
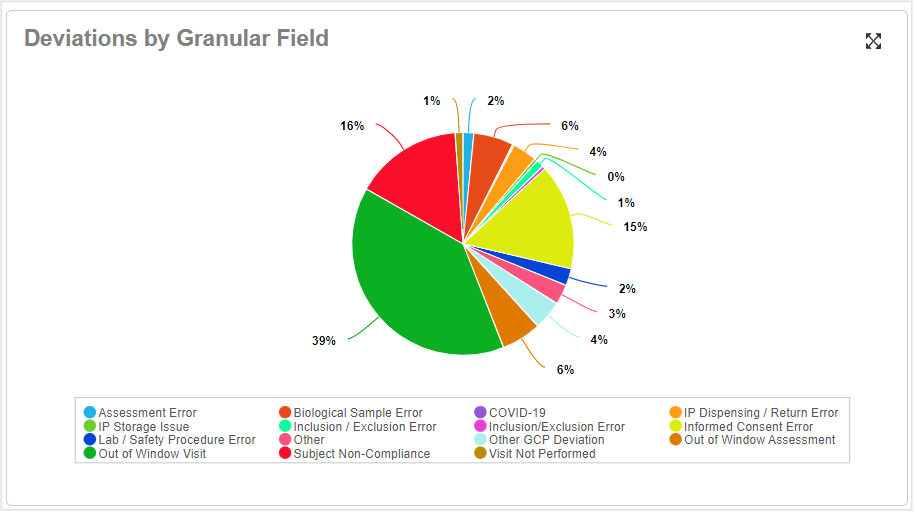
Figure 3: Deviations by Type
Being unable to show proof of oversight compliance significantly increases risk and can jeopardize the success of your study. Learn about the technology you should use to develop a scalable, compliant framework for outsourced studies.