Blog
Top Three Drivers to Improve Study Start-up
Aug 14, 2019 | Ashley Davidson
Aug 14, 2019 | Ashley Davidson
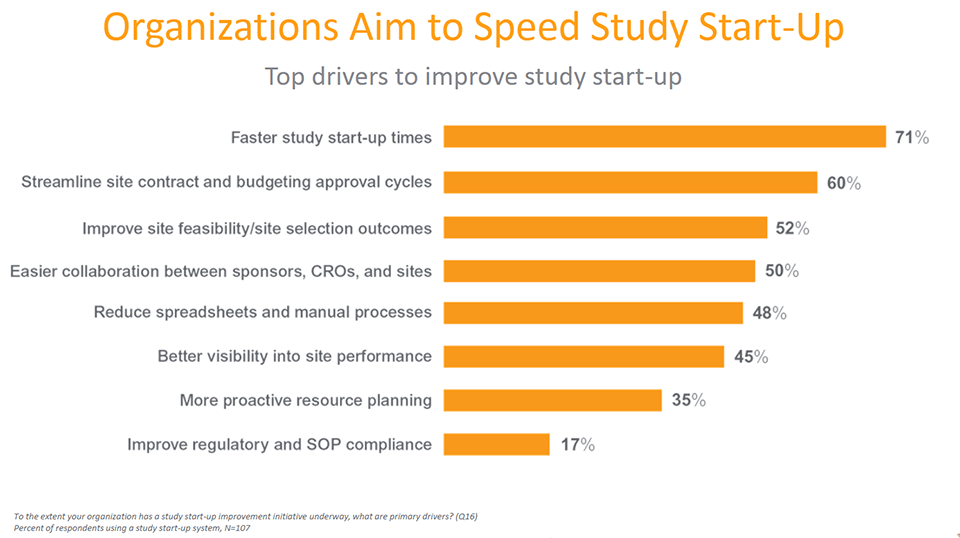
Study start-up has significant impact on the overall speed of a trial, making it one of the most critical parts of the study process. Yet the resource-intensive study start-up phase of clinical trials is no faster today than a decade ago. In a recent industry-wide survey, all respondents report challenges with study start-up – likely due to the heavy reliance on manual processes, since 81% report using spreadsheets as their primary study start-up tool. The prolific use of spreadsheets is a challenge because manual methods slow down processes and limit visibility into study status.
Encouragingly, adoption of study start-up applications is on the rise. Nearly one-quarter of respondents now use newer, purpose-built study start-up applications to speed cycle times. Streamlining study start-up enables trials to run more quickly, speeds site activation, and allows patients to be enrolled faster, ultimately speeding drug development.
For an overview of current start-up approaches and top areas for improvement from our survey report, watch this 8-minute video.