Blog
Using Data Analytics to Improve CRO and Sponsor Partnerships
Jan 18, 2022 | Jennifer Kratz
Jan 18, 2022 | Jennifer Kratz
As global drug safety regulations become more stringent, pharmacovigilance teams are under increasing pressure, not only to process a rising number of incoming adverse events, but to actively monitor adverse events from an expanding number of sources, as early as possible.
To meet these challenges, a growing number of companies are automating certain functions and using more advanced technology. They are also outsourcing more pharmacovigilance activities to service providers and contract research organizations (CROs) like NAMSA. While outsourcing any strategic function involves risks, with the right solutions and data-driven approach, it’s possible to improve results for both sponsors and CROs while avoiding common challenges.
NAMSA is using real-time analytics in Veeva Vault Safety to improve collaboration with sponsors, driving positive change through greater transparency and tracking key performance indicators (KPIs) in four areas: operations, vendor oversight, regulatory compliance, and vigilance.
Need for real-time transparency
In any outsourcing relationship, trust is essential for both parties. To achieve it, sponsors and CROs need to co-author safety management plans and detailed work instructions so that CRO staff understand expectations, limitations, and business requirements. CROs must also share information and work collaboratively with sponsors on the approach and processes they use and provide information on real-time reporting capabilities and data analytics.
Proactive CROs monitor and track projects closely to address challenges and mitigate risks. This approach helps them ensure they have assigned sufficient resources to each project, and they are performing as expected. Better alignment with sponsors requires CROs to keep a close watch on resource use and team efficiency, and that they understand how well they are meeting sponsor expectations (via KPIs), requirements per agreements and plans, and protocols. Close alignment builds trust and transparency and requires a foundation based on real-time operational reporting.
How data analytics can help
At NAMSA, we’ve addressed these challenges by creating Vault Safety dashboards and reports that monitor four key areas:
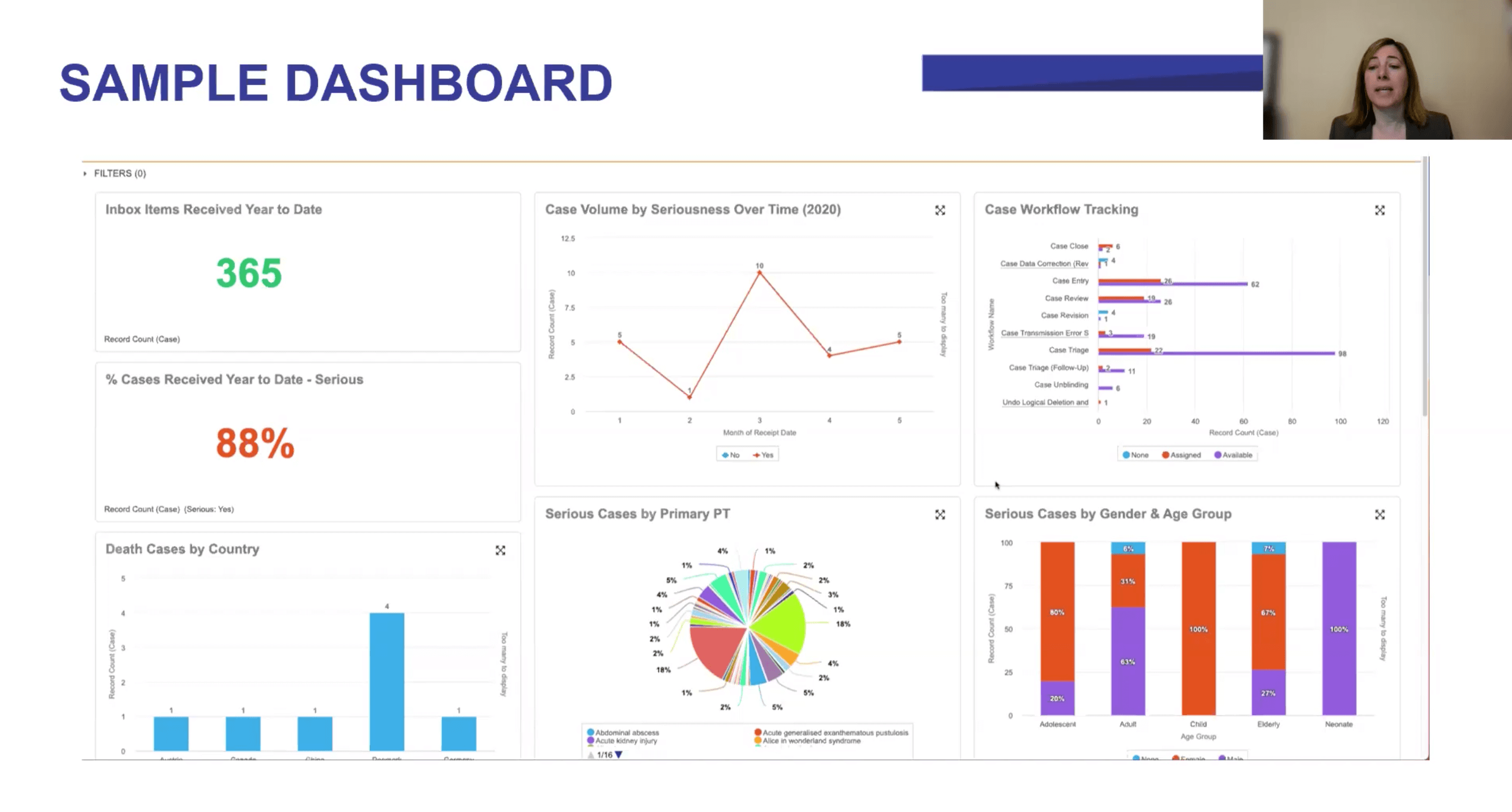
- Operations
Operational information can show how each client’s project is progressing, and this in turn helps NAMSA keep a close eye on resource usage plans. For example, we can make projections based on case volume or complexity trends to ensure the right number of resources are in place to meet demand and reallocate resources quickly if needed.
We also use data to gain better quality oversight. With Vault Safety, we can allocate and track quality control tasks to people across the safety organization. For example, quality review of laboratory data entry can be assigned to one team member while another checks the narrative for accuracy.
- Vendor oversight
NAMSA has also established analytics tools to monitor staff performance and ensure we are providing the right resources and support to each contract. Overall trends give our sponsors the big picture for their projects, but they can also drill down to check individual data points, such as KPIs.
The “Vault Safety Action Item” report is useful for providing information on each work task for a case: when it was created, who created it, who it is assigned to, and for how long it has been open. This also allows the CRO to make sure that tasks are completed on time.
- Regulatory compliance
A compliance dashboard shows the rate of SUSARs (suspected unexpected serious adverse reactions) as well as the number of expedited versus non-expedited cases. This also makes it easy get the line listings for aggregate reporting directly from Vault Safety. Regulatory affairs staff are given access to the database so they can view or run reports and update RMPs/REMS as needed.
- Vigilance
Medical staff who are overseeing patient safety can also leverage Vault Safety to discover trends in medical history, laboratory data, or adverse events across patients or sites for a study. For example, they can use demographic data or patient population, such as pregnancy or age, to gain insights into product usage.
Win-win for safety
By using real-time analytics and dashboards, both sponsors and CROs can expect to see a myriad of benefits. For one, it increases transparency between the CRO and the client, in turn building trust and promoting better alignment. It also improves efficiency; no one struggles to find the information they need because it’s all readily available.
As we say at NAMSA, “Safety doesn’t sleep.” Sponsors and CROs need to be in sync if they are to meet increasing demand and improve drug safety. Vault Safety offers all stakeholders the opportunity to harness real-time data visibility with reports and dashboards so that users easily get the information they need to support better decision-making.
Providing transparency improves trust and deepens collaboration between sponsors and CROs. At NAMSA, it has allowed us to improve efficiency and work together with clients to meet pharmacovigilance challenges head on.
To learn more, watch the Clinlogix/NAMSA presentation at the 2021 Veeva R&D and Quality Summit, North America, by visiting veevaconnect.com.