Blog
What’s Driving Clinical Data Innovation Now: Notes from the Front Lines of Change
Dec 05, 2024 | Manny Vazquez
Dec 05, 2024 | Manny Vazquez
From April to November, clinical data leaders met at separate forums in Basel, Switzerland, New York City, NY, London, England, Copenhagen, Denmark and San Francisco, CA. They discussed the trends that drive innovation, initiatives now underway, their likelihood of succeeding, and future requirements. Figure 1 shows the areas that attendees think will provide the most value and the highest near-term probability of success.
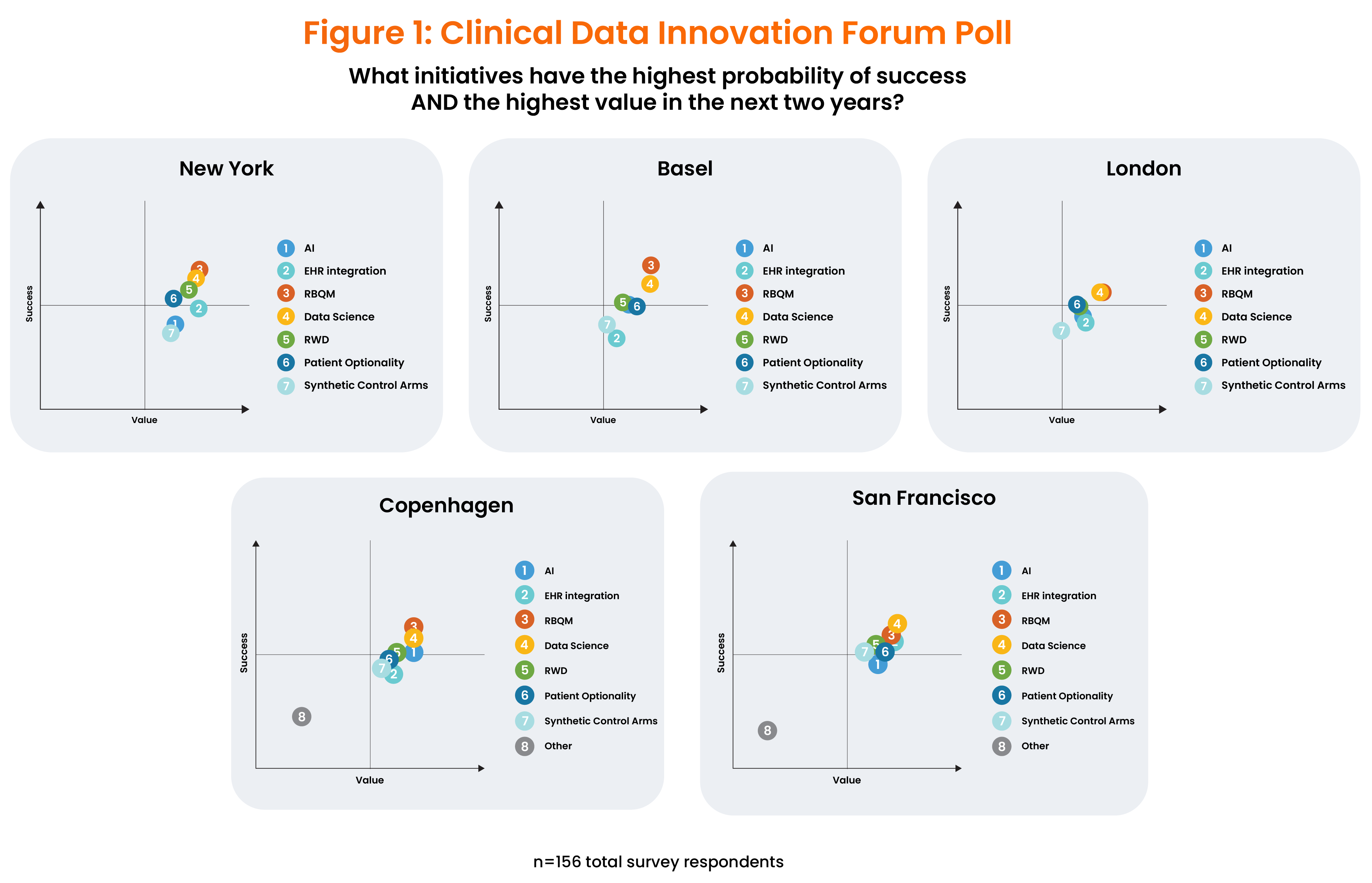
Even 4,000 miles apart, attendees aligned on the near-term drivers of innovation. Despite lingering hype surrounding artificial intelligence (AI), leaders have mixed feelings about it, acknowledging that it will help support clinical data science but giving it a low rank in terms of its likelihood of succeeding in the near term.
Instead, biopharma companies are prioritizing risk-based quality management (RBQM) – and more specifically, risk-based clinical data management (RB-CDM) – as their #1 near-term innovation driver. These approaches are also supported by recent regulatory guidance, and in fact, SCDM is currently writing an RB-CDM chapter for the GCDMP to explore this further. As Hany Hanalla, a senior manager, clinical data management, and a panelist in NY said, “We need to stop babysitting data that doesn’t matter.”
At the same time, clinical data teams increasingly see clean, harmonized clinical data as a “product” for downstream groups and themselves as the marshals of this data. This represents a shift into clinical data science, which poses new challenges, attendees noted. As more sources of patient data come into play, there will be more consumers for this data, and teams will need to manage the movement of that data even more closely than they do now. Developing standards is a top goal for data management, which can streamline a centralized, strategic approach.
More clinical data teams at sponsors and CROs have also realized the importance of working more closely with research sites to understand other sides of the Rubik’s Cube and follow the rule of never talking about the site without the site in the room. This effort promises to increase trial efficiency by improving the research site end-user’s experience. At the same time, working more closely with those closest to patients will offer clinical data and operations teams deeper insights into how trial design, protocols, and operations affect patients and what patients expect from each trial.
RBQM is the top priority
Teams must consider risk carefully at every stage of each clinical trial. Some are still relying on source data verification (SDV) until they feel more comfortable with risk-based monitoring and RBQM. However, moving to RBQM has become an industry priority. “We need to stop cleaning low-risk data and relying on SDV to become more efficient, and make decisions that are fit for purpose,” commented one data leader. As they work with RBQM, more clinical data teams see audit trails as an important way to understand which data — whether on operations, site performance, patient safety, or clinical endpoints — is most critical to determining risk.
At the London event, a poll conducted by Leonie Christianson, a business consultant at Syneos Health, showed that almost half of the attendees have adopted RBQM methodology. Many acknowledged that activities like listings review and non-critical data checks persist, highlighting the need for modernizing data review skillsets, processes, and a solid technology foundation.
Industry-wide standardization with automation
Biopharma companies are taking different pathways to standardization. Some are opting to maintain complete control of their trial data, but outsourcing study builds. Others are integrating CROs into their data management operations, using a functional service provider (FSP) model. At all forums, attendees discussed the goal of establishing end-to-end industry-wide standards for process and system interoperability, as well as standard libraries for common forms vs. therapeutic-specific forms.
Closer collaboration is needed with sites and patients
At the Basel event, experts shared and discussed the challenges that clinical research site staff often experience with new applications from sponsors and CROs. Speakers emphasized the need for all stakeholders, as well as technology vendors, to understand the site’s experience—and with it, the patient’s—for any new application or tool. Currently, few sponsors routinely involve sites in study design and setup phases (i.e., protocol and systems review).
Early on in each trial, research staff typically focus on EDC and eCOA, but, as the trial progresses, they must master more applications and solutions for eConsent, eSignatures, and payments. As a result, at many research sites, staff end up working with 15-20 applications per study, managing multiple spreadsheets manually, and coping with passwords that are updated every 6-8 weeks. As one speaker asked, “Can a site generate quality data if its staff must enter the same data many times in different systems?
Vivienne Van Der Walle, MD/PhD, who leads the independent research site PT&R, shared at the London event that the first patient in a trial is a “trial within a trial” due to complex systems. Every sponsor has its own definitions, standards, and preferences for how to set up databases. Without more intuitive, holistic applications and automated processes, patient care and data quality will suffer, she said. Could there, one day, be one manual for sites?
Van Der Walle suggested that vendors make new applications, such as eConsent tools, more user-friendly and that site end-users and patients be involved in user acceptance testing. Experts also stressed the need for vendors to help site staff at the point of use and end the traditional practice of maintaining one single login, manual, system, and contact point per site. For more insights into how communication and collaboration are being improved, tune into interviews with Vivienne Van Der Walle as well as with Helen Shaw, co-founder of the UK clinical research site, VCTC.
Speeding the shift from data management to data science
Five years ago, the Society for Clinical Data Management first noted the need for biopharma companies to embrace a more scientific approach to clinical data, and move clinical teams from managing data to applying data scientifically. Adopting this approach requires that data scientists become more involved in early trial design and protocol development. It would also enable greater trial efficiency and help speed patient access. Attendees noted that the shift from data management to data science is already well underway, but pointed out the need to set new goals for the future.
They emphasized the need to focus on KPIs and performance targets at the start and end of each study to improve speed while maintaining quality, and believe that acceleration must occur across the following pillars:
- Optimized patient data flow
- Integrated data quality and review
- Use of AI/ML and advanced analytics
- Evolved roles and skill sets
- Digitized and automated analysis
Embracing the move to data science will also require new skill sets within clinical data teams. As one speaker noted, “We need to move away from merely checking boxes and into interpreting the data.” Others pointed to the need to think end-to-end, beyond the traditional boundaries that have defined clinical data teams.
Developing a new mindset and trusting new tools
Clinical data professionals will also need to adopt a new mindset and become more comfortable with automation and new tools. “We need to get to the point where we trust the tool. We burn resources by constantly checking our work,” one attendee pointed out.
In the future, increased use of real-world data promises to move the industry away from phased trials toward continuous regulatory submissions, in which every study continues to support claims. Teams will need a clear understanding not only of what data must be collected but also why, and they will also have to ensure that the purpose of the data is well-defined. In addition, the industry must move away from the study-to-study view since “what we are looking for is constantly changing,” as one attendee noted.
Wish lists and magic wands
Among the changes discussed was the future role of EDC, since most of the patient data required for trials today now exists outside its boundaries. In the future, the tool may be used mainly by sites. (For more on innovating beyond the EDC, listen to this interview with Patrick Naldolny, global head of clinical data management at Sanofi).
Forum attendees also provided a wish list of requirements for meeting the industry’s future needs, which included the examples below:
- Standardizing and automating data management so that real-world data can be integrated in a useful way.
- Enabling CROs to move beyond providing data collection as a service, so that they become part of the sponsor’s data science ecosystem, a transformation that FSP contracts are starting to advance.
- Ensuring that clinical data teams have a seat at the table in discussions involving data acquisition, data warehouses, assistance with AI or other approaches needed to support automation.
One of the things I enjoy most about interviewing experts on Veeva’s podcast program is asking guests what they would do if they had a magic wand and the ability to change or stop existing practices or start brand-new ones. Forum attendees responded to that question with the following priorities:
- Stop using SDV
- Start implementing continuous and risk-based data cleaning
- Improve standards management, and establish libraries that can be accessed by all build resources (including CROs, sponsors, and FSPs)
- Improve cross-functional collaboration between clinical data and other teams
- Make data management part of protocol development
- Stop collecting data that isn’t used
- Stop using SAS and, instead, use more AI to handle data
We will be hosting more innovation and other industry forums worldwide to explore trends, challenges, and innovative solutions. To stay informed of our upcoming events, click here to learn more. In the meantime, please check out Season 3 of the Digital Clinical Trials podcast series for best practices and insights.