Blog
Oncology Trial Design and Study Build
Dec 07, 2021 | Richard Young
Dec 07, 2021 | Richard Young
Oncology trials are becoming increasingly complex and require more sophisticated trial designs. They often need to be based on adaptive trial principles. EDC technologies haven’t kept up with these new challenges, until now.
Complex, variable patient journey
Clinical trial durations of oncology drugs are 30%-40% longer than other drug trials, due to more complex designs and difficulty finding, enrolling, and retaining study volunteers (Tufts CSDD, 2021). Unscheduled, flexible, repeat assessments create variability that further complicates design.
In Vault EDC, we have eliminated the need for every variation to be predicted, tested and validated. Productized dynamics automatically add, amend and label the required assessments, based on the study design and the patient journey. With rules and dynamics, fields, forms, visits, or new cohorts can be added dynamically, based on a simple trigger question. This reduces the setup challenge. Across complex studies, amendments will impact different patients in different ways, leading to complex matrices of decisions. Dynamics ensures that this complexity is streamlined, maintaining efficiencies across multiple amendments.
Study schedule editor
Managing complex designs and building multiple unique visit structures are time consuming. Rather than requiring individual matrices for each scenario, Vault EDC provides a schedule editor that works in tandem with dynamics to provide a single view of the complete study. A display closely aligned to the schedule of assessments grid enables study teams to rapidly assess how to optimize study design, and where to apply dynamics effectively. Study teams no longer need to build every potential scenario in advance to avoid amendments.
Custom Functions
In traditional EDC solutions, the functionality needed for oncology trials is rarely available as native capabilities. Study teams are forced to augment the core functionality with additional utilities delivered via custom functions—external custom code written by a programmer. There may be 300-400 custom functions in a single study. These custom functions increase the testing burden during each amendment therefore increase the cost of making amendments.
In Vault EDC there are almost no custom functions, we average five custom functions per study and that number drops with each software release. The rules engine is designed to handle each and every edit check, and can perform functions such as sending email notifications and adding dynamic visits and forms. Additionally, productized functionality reduces the need for writing rules. For example, a date comparison tool replaces scripting rules with point-and-click configuration. Vault configures the business logic behind the scenes – no coding needed. Another example exists with local labs. Traditionally, calculating the patient’s age and matching this with the right local lab range required a custom function per visit. Veeva productized the age calculation at each visit and uses that to automatically assign the correct range. No coding required.
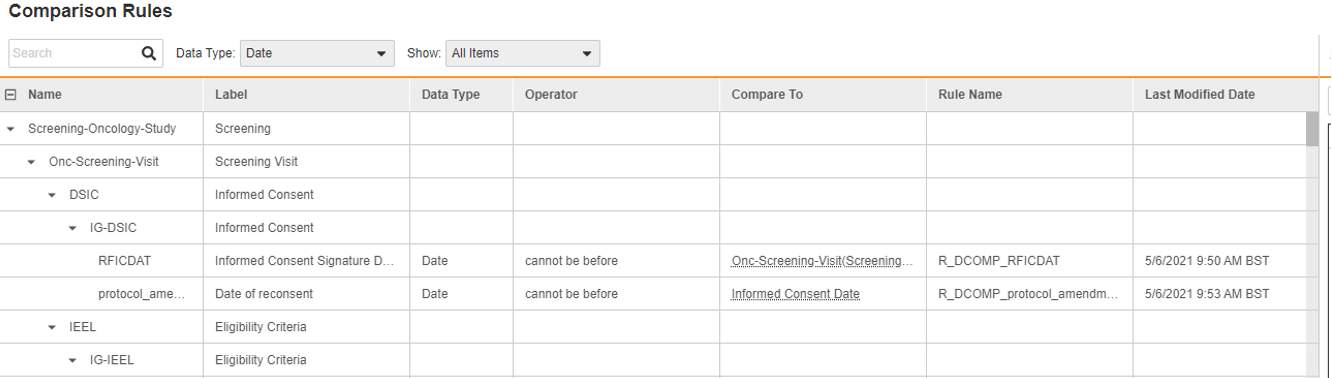 Figure 1. Date comparison configuration interface.
Figure 1. Date comparison configuration interface.
There are many other challenges, but hopefully these three examples illustrate how modern tools for study builds equip you to optimize your trial designs and run the oncology trials you need. Learn how to increase agility in oncology trials with Veeva Vault CDMS.
