Features Brief
Veeva CTMS for Study Oversight Datasheet
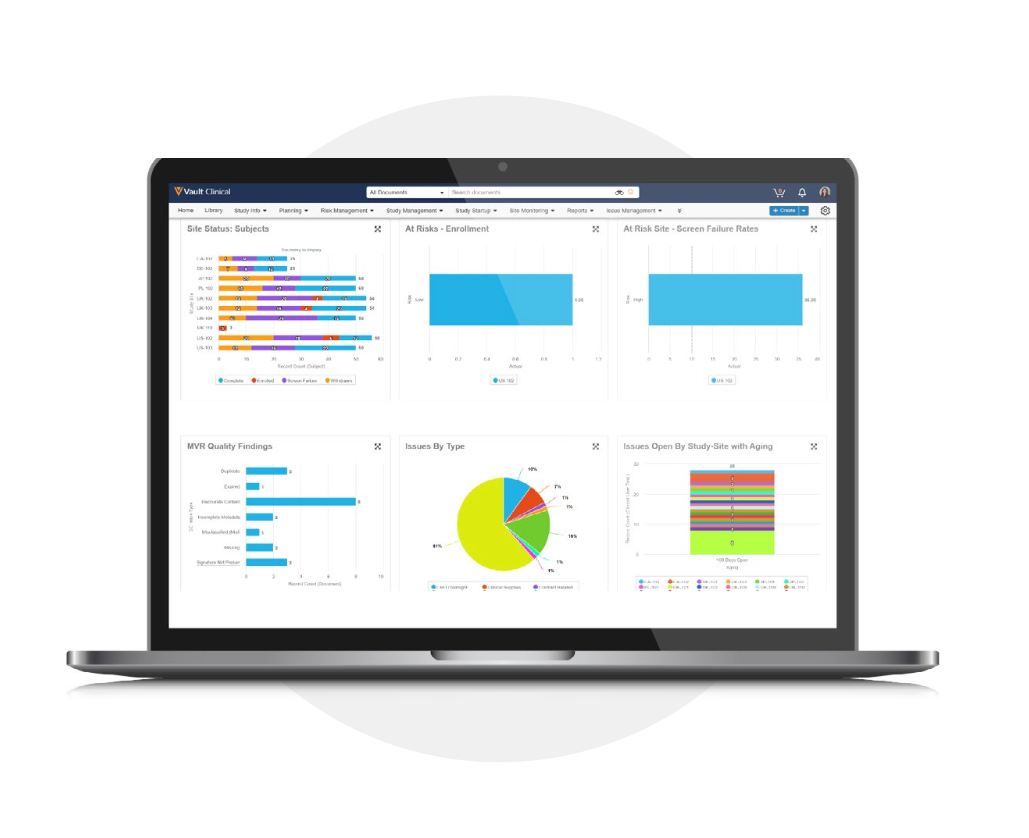
Veeva CTMS for Study Oversight in Outsourced Clinical Trials

Sponsors that delegate clinical trial activity to contract research organizations (CROs) have a regulatory responsibility to ensure patient safety, CRO compliance with standard operating procedures, data quality, and trial integrity. Regulations require sponsors to maintain oversight throughout the course of a study – and inspectors expect to see evidence and documentation of proper study oversight.
Yet many sponsors struggle to manage their trials in an outsourced model because they lack the mechanisms to perform oversight. Manual inconsistent reports in different formats from multiple CROs are ineffective and not timely, hindering study visibility.
Veeva CTMS is a modern cloud application that provides the data, documentation, and visibility to drive trial performance. From actionable insights to closed-loop issue management and protocol deviation triage, Veeva CTMS enables effective study oversight in outsourced clinical trials.
Benefits
-
Fewer inspection findings
Better data and documentation provide evidence of oversight, improving regulatory compliance. -
Faster time to market
Speed trial execution by proactively identifying issues, managing risks, and mitigating timeline slippages. -
Stronger engagement with CROs
Actively monitor trial and CRO performance to improve collaboration and inform decision-making. Get visibility to CRO adherence to service and operational level agreements to strengthen contract negotiations that can result in cost savings that are reinvested back into the business.
Features
-
Controlled Activity: Closed-Loop Issue Management
Veeva CTMS provides full lifecycle issue management, allowing sponsors and CROs to work together to drive towards resolution. Capture, create, and manage protocol deviations, clinical tasks, and follow-up items in one system for full visibility and transparency across study partners. Additionally, communication logs, audit trails, and visibility to document changes provide evidence of reviews and monitoring oversight for complete regulatory compliance. -
Visibility: Real-Time Reports and Dashboards
Operational data is presented in a useful, actionable manner that allows sponsors to drill down to the details. Reporting insights prompt activity on issues and tasks, while role-specific dashboards guide activity by study, program, country, and site.
Sample KPIs and KRIs
- Number of serious adverse events by site
- Patient discontinues and withdrawals
- Missing informed consent forms
- Protocol deviations by site and study
- Time to issue resolution
- Monitoring visit report quality findings
- Unresolved follow-up items over time
-
Collaboration: CRO Integrations
Sponsors and CROs can collaborate and transfer data easily into Veeva CTMS through manual and automated methods. Access multiple data domains, such as patient data, milestones, trial timelines, and issues, for a comprehensive view to keep studies on track. Data exchange from CRO applications to Veeva CTMS has never been easier. -
Engagement: CRO Performance
Monitoring is one of the most costly line items in a study budget, and measuring CRO performance to service and operational level agreements can surface insights that strengthen contract negotiations. Quantifying adherence to SLAs and OLAs can result in favorable negotiations and cost savings that are reinvested back into research and development.
Useful Metrics to Track CRO Performance
- Time to query resolution
- Outstanding queries
- Recruitment plan vs. actual
- % of low-enrolling/no-enrolling sites
- Monitoring timeline adherence