Features Brief
Veeva CTMS Features Brief
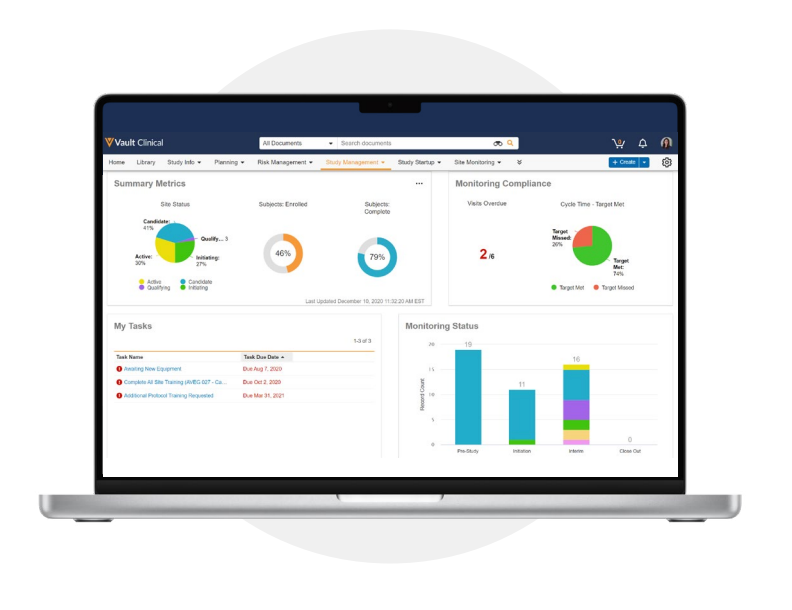
End-to-end trial management with a modern CTMS
Veeva CTMS is the only flexible cloud application that makes it easy to streamline trial management, gain complete visibility across the portfolio, and unify clinical information and processes for insourced and outsourced studies.
Study teams can manage the entire end-to-end clinical trial processes and gain a global view of tasks in one unified and secure system. With simple navigation and a single source for clinical master data and study information, Veeva CTMS improves operational efficiency and enables faster, higher-quality trial execution. Streamline operations with a flexible, agile solution that easily adapts to your organizations's unique clinical trial needs and business processes.
Benefits
-
Increase Productivity and Save Costs.
Streamline monitoring planning and execution to boost efficiency, cutting time spent by over 30% and reducing trial costs -
Improve Decision-Making.
Strategic trial planning is easier with a full view of global operations in a single system. Organize trial execution by actively managing activities, tracking key performance metrics, and mitigating delays in a unified system. -
Enhance Collaboration between Sponsors and CROs.
Monitor portfolio performance to identify trends, drive impactful decisions, and enable proactive actions.
A Single Source of Truth
With Veeva Clinical Operations, study teams enjoy a consistent experience with single sign-on and avoid constantly switching between multiple systems. Submit trial information and documentation once and leverage it across different systems, sites, and countries. This single source of truth improves visibility and control, accelerating trial execution.
-
Study Planning and Management
Plan and track study milestones across insourced, outsourced, and other trial modalities to optimize resources and proactively plan for events such as aligning clinical supply arrival within the site initiation visit or assessing site performance across studies. Veeva CTMS enables seamless subject visit planning, based on categories such as protocol, visit frequency, and procedures. -
Issue Management
easily capture, track, and manage protocol deviations, issues, and follow-up items across all studies. Quickly identify actions needed, assess clinical task statuses, and document quality issues to enable closed-loop issue management. -
Study Oversight
Monitor study performance, report on the progression of subjects throughout the study, track CRO activity, and maintain communication logs to help ensure compliance with ICH/GCP guidelines. CRAs can also capture and track protocol deviations for effective issue management. CTMS Transfer enables seamless data exchange between sponsors and CROs, making oversight easy. -
Subject Recruitment Planning
Plan the number of subjects that will be screened, enrolled, or randomized within a study and get a comprehensive view at the study, study country, and site levels. With metrics that update with actual recruitment data, you can track subject enrollment against goals to ensure studies are on time. -
Investigator Relationship Management
Empower study teams with an accurate and complete view of interactions between sponsors, CROs, investigators, and site personnel. Track site communication logs, site monitoring visits, resources assigned to sites, and more to strengthen collaboration and improve study execution. -
Interactive Dashboards and Reports
Create reports that show time-operational metrics, documentation, and information by study, country, investigator, site activation status, and more. Study teams can take immediate action directly from dashboards, eliminating bottlenecks and increasing efficiency. -
Site Monitoring
Manage all aspects of routine monitoring visits - pre-study, site initiation, interim monitoring, and closeout - in Veeva CTMS. CRAs can view key information such as enrollment metrics and violations at-a-glance on the CRA homepage, quickly author new monitoring visit reports, and track onsite monitoring activities, all in one application. -
Risk-Based Quality Management
Reduce operational risk and improve data quality with configurable risk assessments. Define critical data and processes, calculate risk scores, and implement migrations to focus and keep trials on track. -
Clinical Operations to EDC Connection
Eliminate duplicate data and transcription errors and provide real-time visibility into enrollment status at every site. Study managers and CRAs can move seamlessly between subjects or subject visits in Veeva CTMS to the applicable events and forms in Veeva EDC without a separate login. -
Site Payments
Unified with Veeva CTMS, Veeva Payments speeds payments to clinical research sites, tracks study budgets, and provides transparency to payment status. Designed to support complex trials, Veeva Payments enables sponsors and CROs to pay sites faster and more accurately. -
Clinical Operations to CRM Connection
Improve coordination, efficiency, and transparency between study teams in Veeva Clinical Operations and medical science liaisons (MSLs) in CRM by transferring healthcare provider (HCP)-related activities.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution. Streamlined processes and improved data visibility from startup through closeout accelerates timelines and enhances collaboration across sponsors, sites, and CROs.