Article
Deploying a Digital-First Field Team
for Hybrid Engagement
THOUGHT STARTER SERIES
The healthcare professional (HCP) of today is a digital citizen with greater control, changing needs, and evolving expectations. Individual HCPs have varying preferences, and certain segments are now turning first to digital channels as key sources of information. As such, life sciences companies must be flexible in how they engage with HCPs based on customer preference and business need.
The explosion in digital engagement brought on by COVID-19 has shown doctors a convenient and efficient way to engage with reps remotely.
As a result, nearly 90% of HCPs now want to meet reps through hybrid or all virtual methods.1
Field forces are now under pressure to deliver consumer-grade digital experiences to complement face-to-face interaction. But considering many reps have spent the bulk of their careers engaging in person, they face a challenge when it comes to capitalizing on these new methods of engagement. Here’s how and when to use a digital-led engagement model to complement existing in-person communication.
All stakeholders benefit from the ability to blend in-person and digital engagement, including HCPs and life science organizations. Many companies report seeing:
Improved customer experience Providing the option for both remote and physical engagement means companies can meet customers on their terms.
Expanded access Reducing the reliance for physical access creates greater flexibility in how and when reps access HCPs.
Greater personalization Digital engagement means more data, providing opportunities to create a more tailored and impactful experience.
Higher productivity Field forces can use time saved travelling to increase the volume and reach of touchpoints.
When digital-led engagement makes sense
With HCPs’ growing preference for virtual engagement, some companies are starting to experiment with teams of reps specializing in digital that sit alongside traditional or hybrid reps. The focus of these digital teams could be based on customer need (e.g., HCPs who predominantly prefer digital interaction) or on business need (e.g., in cases where efficiency is needed at certain stages of the product lifecycle).
However, ‘digital first’ does not, and should not, be the mantra for every interaction with customers. Delivering an exceptional customer experience is only achieved when reps continue to focus on nurturing HCP relationships—offering the right content, through the right channel, at the right time to have the biggest impact. What is ‘right’ must consider both customer as well as business requirements.
Thus, the answer to the ‘right channel’ is not always going to be a digital one. Here’s how to figure out when, where, and how to use digital engagement:
1. Reflect on any existing insights on customer preference: Before meeting with the customer, assess initial data on known preferences or segmentation to route customers first to either a digital rep or a field rep from an adjacent team for a face-to-face meeting.
2. Balance with business need: Once the lead has been qualified and scored through the first meeting, use a pre-defined set of criteria to decide on the optimal customer routing, considering factors such as customer preference, customer value, or product lifecycle stage. Questions to help tailor this framework for your own specific needs can include:
- Should lower-value customers be engaged with predominantly through digital channels regardless of preference, in order to increase the potential of our coverage?
- Do we lean towards digital engagement for mature products, moving towards loss of exclusivity, in order to drive efficiency?
- Will we use face-to-face meetings more heavily for new launches, new HCP relationships, or highervalue customers?
- What are the triggers within a digital engagement flow that would mean we should move that customer into an engagement model with more face-to-face interaction?
3. Optimize engagement: As your understanding of preference grows, you can further narrow down the customer’s preferred channel (e.g., if engaging remotely, is there a preference for outbound or inbound calls, click-to-chat options, or webinars?).
Digital reps are data-driven, technology-enabled salespeople who have mastery of channel optimization and content personalization. Here are the skills and behaviors required by these individuals:
DATADRIVEN & TECH SAVVY
These reps will leverage technology, data, and customer insights to drive evidence-based actions.
CONTENT CURATION
Content forms the foundation of great customer engagement, as it is the vehicle for your message and value to be delivered. Digital reps will leverage a wide arsenal of content to drive personalized experiences.
CHANNEL MASTERY
The digital rep will be an expert orchestrator across all channels, have a strong understanding of relevant use cases, and an ability to integrate and automate their approach.Structuring a digital engagement team
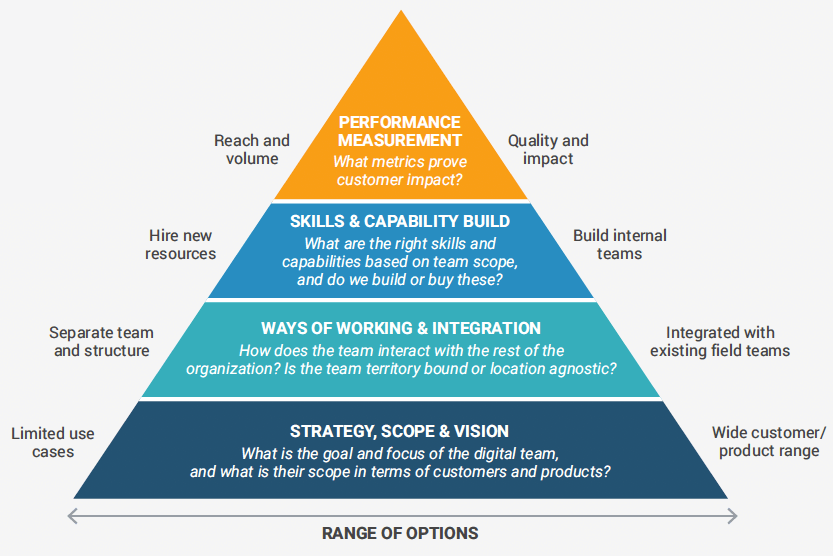
The dynamics of integrating a team of digital specialists into the field force are different from operating traditional teams, and there are some important considerations when designing the right model. This includes the scope and strategy for the team, desired skills and capabilities, ways of working with other field force teams, and performance measurement.
First and foremost, there must be a leadership-backed strategy for the digital team, as well as clear buy-in and alignment with the organizational needs, brand strategy, and the desire to build digital capabilities.
Also make sure to articulate a clear vision and definition of the team’s role and responsibilities. For example:
- Will the digitally focused team be deployed to support the entire product portfolio or only mature products?
- Will this model be used for both commercial and medical field forces?
- Will it be territory-bound or geography-agnostic?
Once the strategic intent and scope has been decided, it’s time to make decisions about workflow and integration. For example:
- What’s the best way to integrate the digital team with existing teams in terms of lines of reporting and management structure?
- Will they have sole ownership over a certain set of customers or shared responsibilities?
- How will hand-offs and interactions with other field teams work?
Another consideration is whether to outsource or build the skills and capabilities in house. This is not a simple question to answer, and it will likely end up being a mixture of both to ensure fresh skills injection as well as continuity.
Finally, with the build of the new team comes the need to measure performance and optimize the team moving forward. Think about:
- How to measure the quality of customer engagement and experience delivered
- When and how to communicate and share insights with existing teams
- How to assess the performance across digital and traditional teams for certain customer segments to inform future engagement strategy
The ideal would be moving away from measuring success purely on digital reach and frequency, and focus instead on producing actionable insights based on metrics around customer satisfaction and loyalty, as well as HCP content, channel, and engagement preferences.
Key decisions must be made on how to create and structure a digital team, ensuring it meets the organization’s strategy and goals.
Hybrid engagement for better outcomes
Capitalizing on digital capabilities is becoming pivotal to driving exceptional customer engagement across all industries. One way of doing this is through building and embedding a digitally led field team that sits alongside and works in harmony with traditional field teams.
These skills can be used to complement physical, in-person interaction, and to create sustainability and scale for wider coverage of customer bases. When executed effectively, this blend of engagement will optimize customer engagement, drive more efficient use of field teams, and ultimately, provide better outcomes for patients.
Key takeaways
Individual HCPs have differing needs and expectations, but increasingly these involve the desire for consumer-grade digital experiences to complement face-to-face interaction.
Most of the field force will need to remain hybrid, but in certain circumstances, teams of digital-only specialists can be used to increase impact and efficiency.
Success in digital interactions will provide a clear point of differentiation, delivering improvement in customer satisfaction as well as internal productivity. But this will only be achieved by building the right people, process, and technology capabilities.
There is no one-size-fits-all approach, and regardless of the channel and method of engagement, a personal touch will always be required to deliver an exceptional customer experience.
Nick MacLeod
Business Consultant
nick.macleod@veeva.com
Fowey Harvey
Business Consultant
fowey.harvey@veeva.com
1 “Is COVID-19 altering how pharma engages with HCPs?” Accenture, August 2020.