Features Brief
Safety-EDC Connection Features Brief
Streamline Clinical Serious Adverse Event (SAE) Reporting and Management
Automatically exchange comprehensive information on reported events, including serious adverse events (SAEs), and streamline reconciliation between data management and pharmacovigilance teams with the Safety-EDC Connection. There is flexibility to choose and transfer information from over 200 casebook and custom data fields (100% more than E2B), including relevant subject data. Easily sharing applicable information reduces queries for clinical sites and enables pharmacovigilance teams to more rapidly process cases and improve quality of safety outputs.
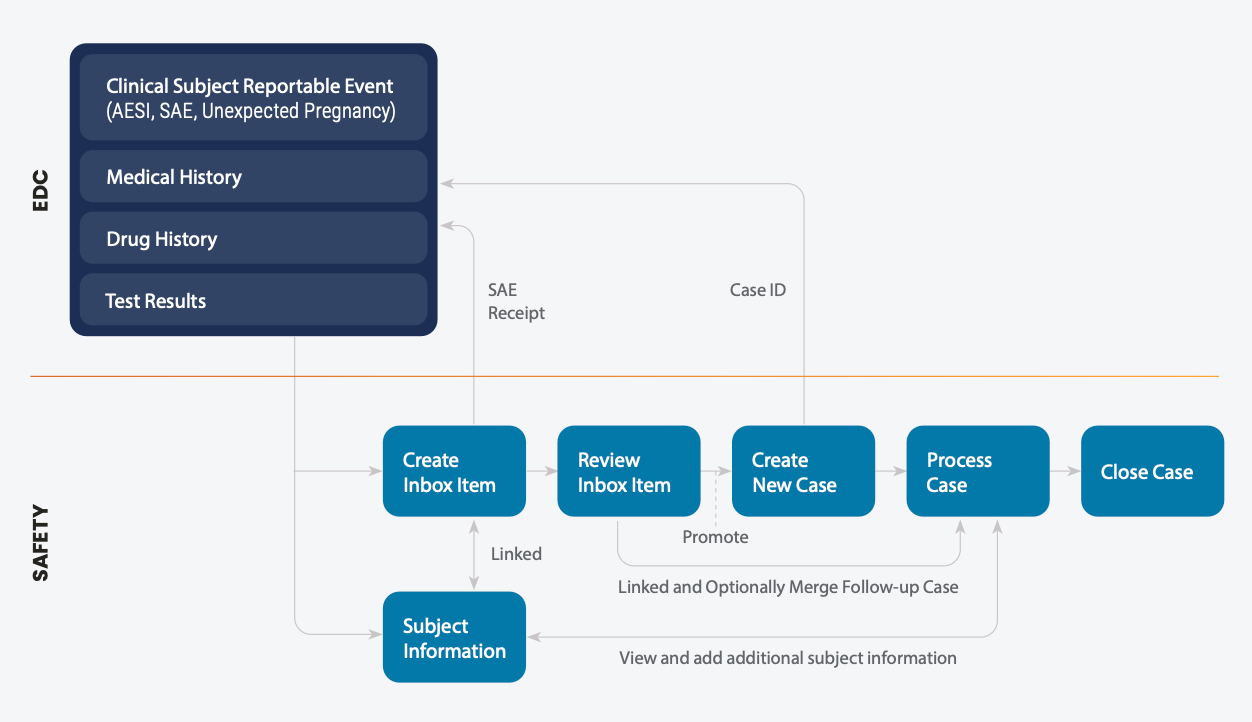
Clinical and data teams have visibility into receipt of SAEs and case ID if an adverse event is promoted to a case in Veeva Safety. If a site(s) makes a change to a SAE, new data is automatically sent to Veeva Safety and linked as a follow-up. Follow-ups may also be flagged as ‘significant’ to ensure timely processing for inflight cases. Linking SAEs and case follow-ups back to clinical source data, reduces risk of inconsistent data and case processing review time as well as streamlines reconciliation processes.
Business Benefits
Veeva’s purpose-built Safety-EDC Connection automates data sharing between Veeva EDC and Veeva Safety.
Safety Team
-
Automate intake of comprehensive data:
Transfer 200+ clinical subject reportable event data fields
-
Accelerate case processing:
View and add relevant subject data
-
Improve safety outputs:
View the SAE with totality of subject information Clinical and Data Teams
-
Streamline reconciliation:
SAE, subject data, and follow-ups are linked back to clinical source
Clinical and Data Teams
-
Significantly reduce manual reconciliation:
Data consistency between EDC and safety applications with automated data flow
-
Reduce site burden:
PV teams independently access and add relevant subject data, reducing queries for sites and clinical teams
-
Efficient communication:
Visibility into SAE receipt and safety case ID
How it Works
When a clinical site enters a SAE in Veeva EDC, the Safety-EDC connection automatically creates an inbox item in Veeva Safety and transfers relevant subject data. A case processor reviews the new safety inbox item, and if it meets the criteria, promotes the SAE to an initial case and shares the Veeva Safety Case ID with Veeva EDC. During case processing in Veeva Safety, if medical reviewers need more data, they can view and include additional subject information in Veeva EDC such as medical and drug history, test results, and other adverse events. If a site user later updates the SAE in Veeva EDC, the Safety-EDC connection automatically creates a new follow-up inbox item, links it to the safety case, and may flag as significant based on pre-defined criteria. The case processor can review and optionally merge the follow-up data with the existing case.
Synchronization of data between Veeva EDC and Veeva Safety is managed and maintained with mapped data fields. Organizations can easily add, modify, or disable default fields, simplifying setup and configuration.
Watch the Safety-EDC Connection demo.