Targeted Pre-Launch Scientific Outreach Drives 40% Faster Treatment Adoption
New Veeva Pulse findings show congresses and early-career experts
have the strongest influence on patient outcomes
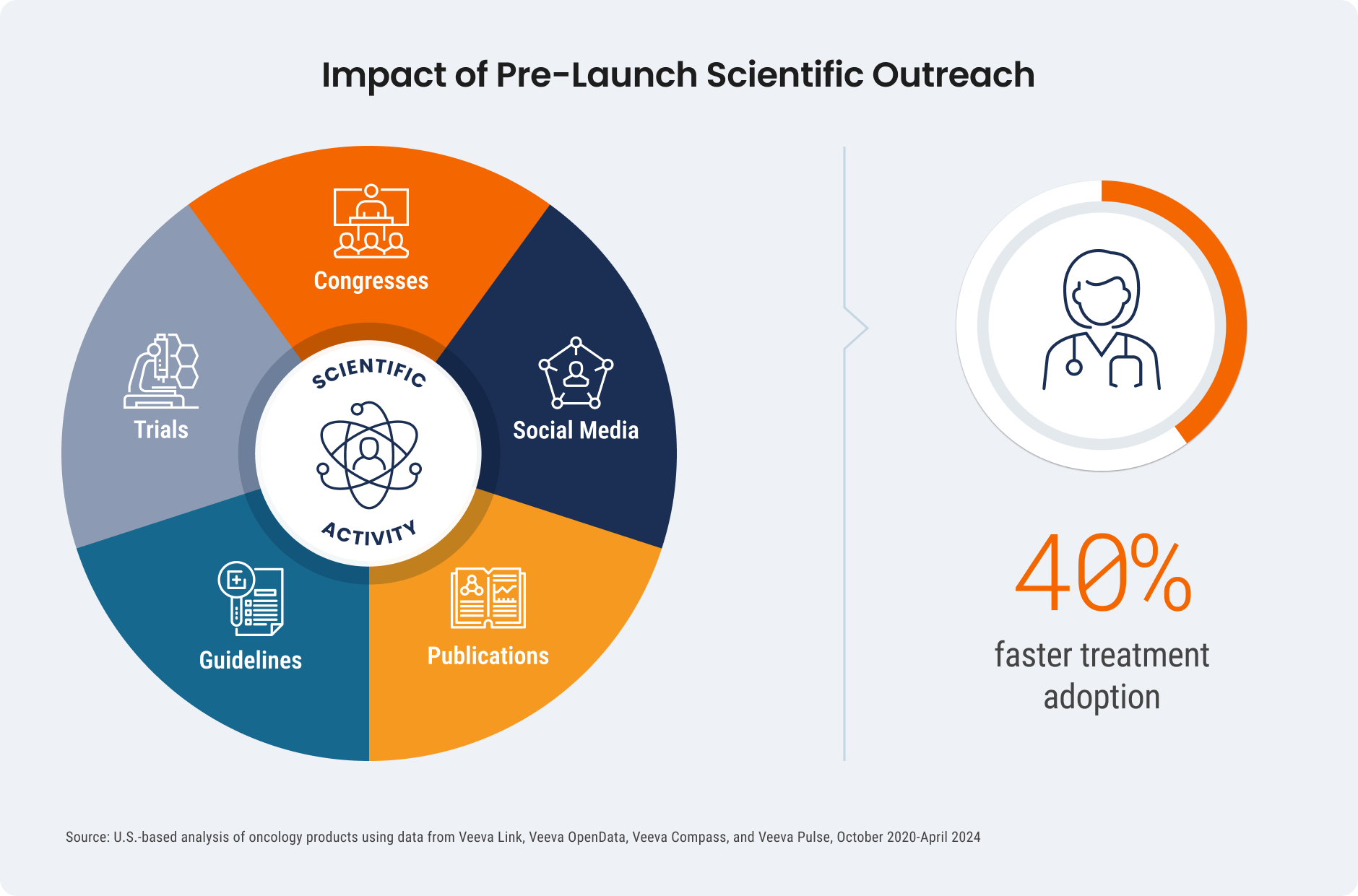
PLEASANTON, CA — July 25, 2024 — Veeva Systems (NYSE: VEEV) today revealed that targeted pre-launch scientific outreach maximizes medical affairs teams’ impact on treatment adoption in the latest Veeva Pulse Field Trends Report. The analysis shows biopharmas investing in pre-launch scientific activities and prioritizing congresses gain 40% faster treatment adoption than those that invested less. Early-career experts are also more open to digital engagement and four times more likely to start patients on a new treatment.
Healthcare professionals (HCPs) are turning to biopharmas to help distill rapidly evolving scientific evidence around new innovative medicines and complex diseases. By focusing pre-launch activity around clinical guidelines, publications, and congresses with a new group of stakeholders, medical affairs teams can reach key HCPs to improve treatment decisions.
“Gone are the days when medical could just focus on the top-tier scientific thought leaders,” said Angela Smart, director of global medical excellence and operations at ADVANZ PHARMA. “The range of stakeholders has broadened, and it’s imperative to expand our engagement strategies beyond traditional experts.”
The latest analysis from Veeva Pulse finds that:
- Targeted pre-launch scientific activities speed treatment adoption: By engaging key experts at congresses, helping develop clinical guidelines, and securing coverage in publications before launch, companies see 40% faster treatment adoption than those that invest less in this outreach. This demonstrates that more resources for medical teams during this stage can boost launch success.
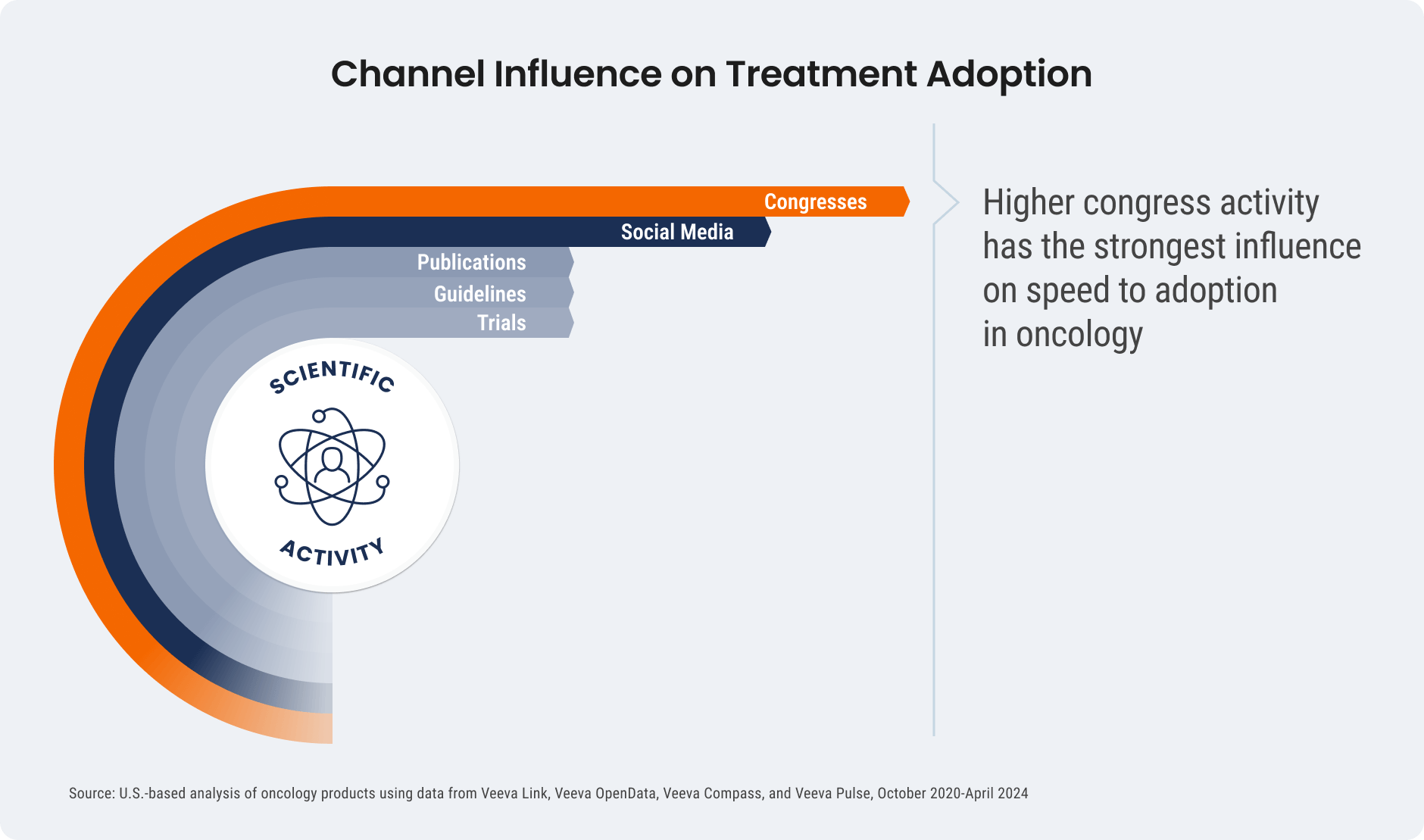
- Congress activity has the strongest influence on clinical decisions: Higher investment in pre-launch congress activities has the most influence on treatment after approval. Congresses, and the digital discussions that follow, help increase scientific awareness and drive impactful medical conversations more than investment in clinical guidelines and publications.
- Engaging early-career HCPs increases new treatment uptake: These digitally-savvy emerging experts are four times more likely to start patients on a new treatment. They are also five times more likely to speak at congresses, 11 times more likely to engage digitally, and seven times more likely to get published.
“As HCPs gain an increasing amount of information to understand complex new medicines across channels, effectively communicating scientific evidence before launch can make all the difference,” said Dan Rizzo, vice president of business consulting at Veeva. “By investing in different types of scientific outreach and engaging newer experts during this crucial window, medical teams can drive greater impact and faster treatment adoption.”
About the Veeva Pulse Field Trends Report
Analyzing over 600 million HCP interactions and activities annually from more than 80% of commercial biopharma field teams worldwide, the Veeva Pulse Field Trends Report is the largest industry benchmark of its kind on HCP engagement. The analysis compiles real-time transactional data recorded in Veeva CRM and Veeva data products to deliver a view of engagement activity across life sciences. Indexed by Veeva quarterly, the data will help companies effectively and accurately benchmark performance to set the right, actionable goals for continued growth and impact.
Additional Information
To download a copy of the Veeva Pulse Field Trends Report, visit: veeva.com/FieldTrends
Learn more about Veeva Business Consulting: veeva.com/BusinessConsulting
Connect with Veeva on LinkedIn: linkedin.com/company/veeva-systems
About Veeva Systems
Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world’s largest biopharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com.
Veeva Forward-looking Statements
This release contains forward-looking statements regarding Veeva’s products and services and the expected results or benefits from use of our products and services. These statements are based on our current expectations. Actual results could differ materially from those provided in this release and we have no obligation to update such statements. There are numerous risks that have the potential to negatively impact our results, including the risks and uncertainties disclosed in our filing on Form 10-Q for the period ended April 30, 2024, which you can find here (a summary of risks which may impact our business can be found on pages 35 and 36), and in our subsequent SEC filings, which you can access at sec.gov.
###
Contact:
Alison Borris
Veeva Systems
925-226-8821
alison.borris@veeva.com