Features Brief
Clinical Operations-EDC Connection
Seamlessly Connect Clinical Vaults to Automate Data Flow
The Clinical Operations-EDC Connection is a Veeva-delivered integration that automates data transfers between clinical data management and clinical operations Vaults, eliminating transcription errors, duplicate data entry, and separate logins. Study teams get better cross-functional visibility without any manual effort, driving efficiencies that speed clinical development.
Business Benefits
-
Simplify setup and maintenance
Speed time to go live by more than 90% with a productized Veeva Connection that delivers bidirectional data without extensive manual configuration.
-
Track and plan recruitment more effectively
Real-time visibility to enrollment and patient status at every site keeps studies on track.
-
Simplify monitoring
Subject visits, SDV requiredness, and new subjects from Vault EDC auto-populate trip reports. CRAs can seamlessly navigate from monitoring visits in Veeva CTMS to casebooks in Vault EDC.
-
Pay sites faster
Procedures and subject visits automatically trigger payable items in Vault Payments without any manual effort, saving time and speeding payments.
-
Improve deviation management
Veeva EDC rules automatically detect and create protocol deviations, then transfer them to Veeva CTMS to centralize deviation management.
-
Streamline reporting
Study country and site data are transferred from Veeva CTMS to Veeva EDC, reducing manual effort and standardizing naming conventions to prevent downstream reporting errors.
How it Works
The Clinical Operations-EDC Connection enables the seamless flow of information by automatically exchanging new records and updates to existing records in a fast, streamlined way through the Veeva Vault platform. Field mappings bridge the terminology differences between EDC and CTMS such as subject events and subject visits.
Configuration flexibility, Veeva’s best practices guide, and user acceptance testing scripts speed validation every release cycle and ensure the connection meets your organization’s requirements.
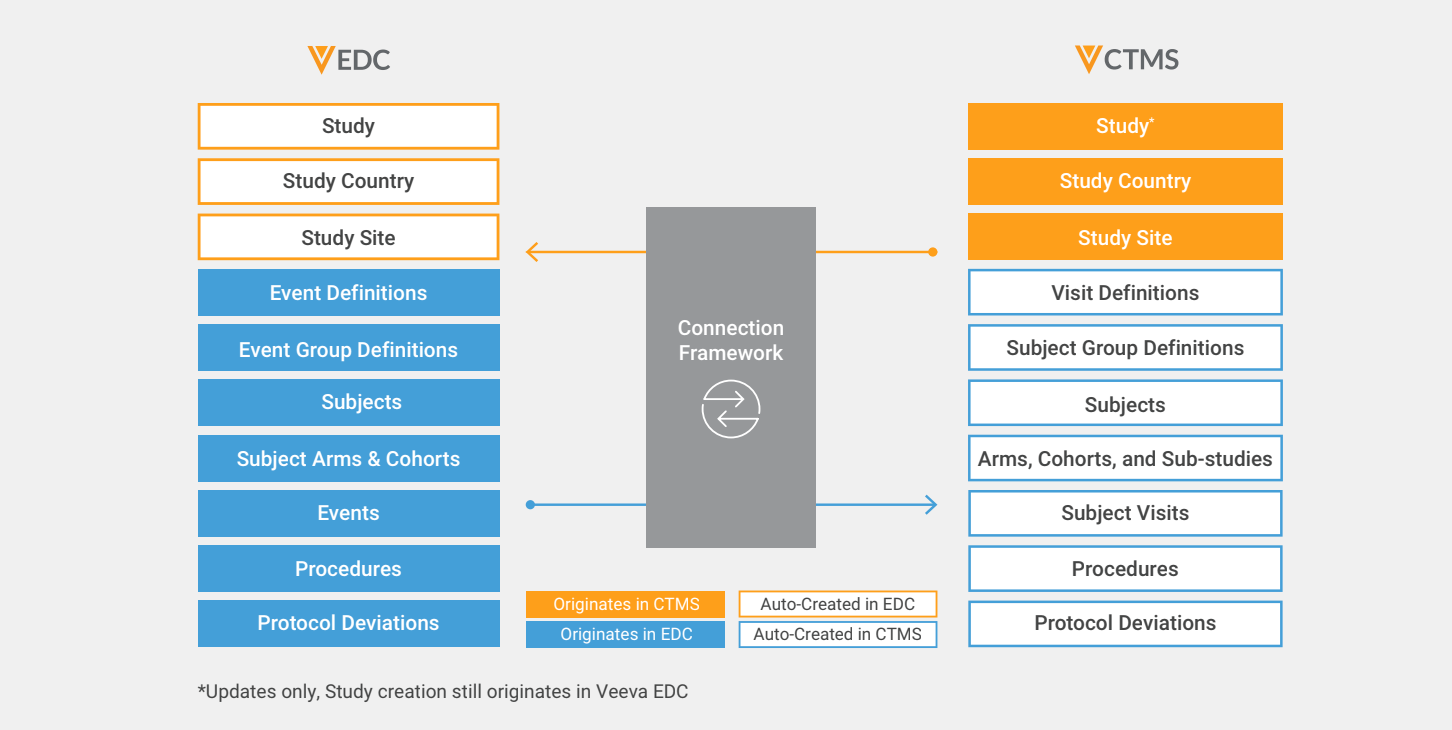
Data originating from Veeva EDC keeps the records and reports in Vault CTMS accurate and current in near real-time. EDC data populates the subject ID, status, key subject milestone dates, and more on the Vault CTMS subject.
For tracking the status of subject visits, data reviews, and SDV, EDC data populates the type of subject visit, status, DMR/SDV review, and more on the Veeva CTMS subject visit.
Configure with Less Effort, Time, and Cost
Third-party integrations require complicated, manually configured integrations and maintenance. The Clinical Operations- EDC Connection is productized and can be configured in just hours, eliminating weeks of integration setup work traditionally required for each study.