Features Brief
Veeva Submissions Features Brief
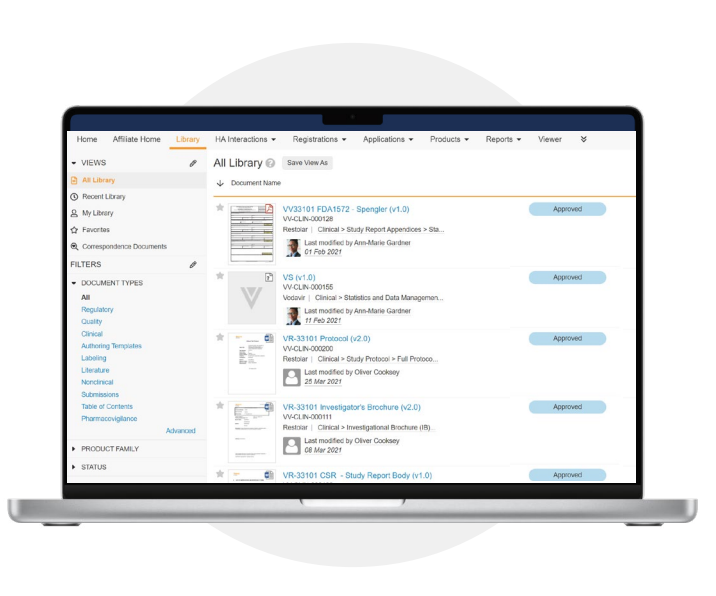
Submission planning, authoring, and assembly
Regulatory information management (RIM) is increasingly complex as new drugs and therapies enter the market with evolving and increasing industry standards. Legacy technologies like spreadsheets and file shares are no longer sufficient to keep pace. And, regulatory teams must identify ways to collect, aggregate, manage, analyze, and act upon a growing volume of data.
Many companies that work with regional affiliates and distributors have difficulty tracking the information sent to local health authorities. Communication with health authorities often takes place via email and status report updates are completed manually.
Veeva Submissions eliminates the need for multiple, siloed tracking systems by providing a single,
authoritative source for regulatory submission content in a secure cloud environment. Companies can manage the
entire submission lifecycle from planning to publishing with greater transparency, collaboration, and control
over their documents and data. Veeva Submissions also allows content creators to securely access and contribute to documents from any location, at any time, and on any device.
When used in conjunction with other Veeva applications, such as Veeva eTMF and Veeva QualityDocs, Veeva Submissions streamlines interactions between departments. Users can reference source materials from other applications, such as clinical documents, manufacturing details, SOPs, and promotional materials. Each department can leverage content as needed while maintaining a single source of truth across the organization.
Business Benefits
-
Streamlined submission management.
Increased visibility into global content assembly and agency submission progress supports stakeholder collaboration, consistent content across markets, and faster submission timelines. -
Faster health authority approvals.
Standardized, automated processes and templates increase efficiency for faster submission timelines. -
Trusted compliance and accuracy.
Improved quality control ensures submission readiness with real-time visibility and automated compliance checks. -
Unified RIM.
Connect end-to-end regulatory processes and improve efficiency as part of the Veeva RIM Platform.
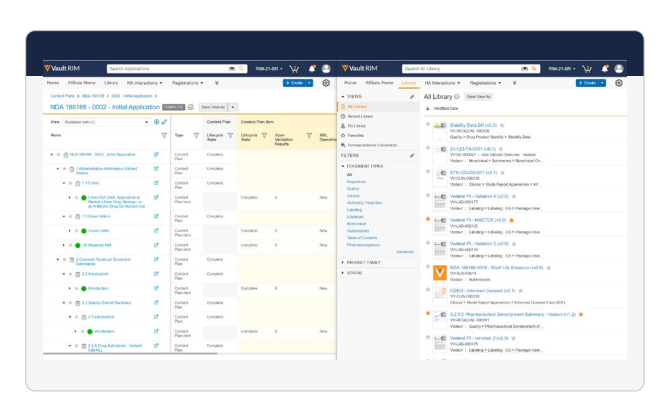
Features
-
Extensible Content Model
Align content taxonomy (document types, subtypes, properties, etc.) with industry best practices, like eCTD and the DIA EDM Reference Model, and extend to meet specific business needs. -
Submission Content Plans
Auto-generate a templated structure for major regulatory submissions, add planned content, and report on
submission status in real time. Also, reuse documents from the library across multiple plans and markets. -
Collaborative Authoring with Microsoft Office
Simultaneously edit documents alongside multiple Vault users while utilizing the full co-authoring capabilities provided by Microsoft Office™. See a demo. -
Global Content Plans
In conjunction with Veeva Registrations, centrally assemble global or core documents related to a multi-market change for streamlined content reuse across local submissions, including those with varying submission structures. Dispatch content iteratively as it’s ready with the ability to compare and review changes before they are committed to a target submission. -
Report Level Content Plans
Compile and publish reports, such as clinical and non-clinical study reports, periodic safety reports,
Investigational Medicinal Product Dossiers (IMPDS), and investigator’s brochures. Create hyperlinks that are
independent of report structure for use earlier in the process during document reviews. -
Robust Lifecycle Management
Replace manual processes with flexible workflows and lifecycles that guide submission authoring, review, and
approval. Authorize individuals to easily change in-process workflows by adding, removing, or emailing participants. -
Interactive Dashboards
Drill down through interactive dashboards to identify the exact source of delays. Take action directly from the reports to address hold-ups quickly and stay on track for submission deadlines. -
Global Access and Collaboration
Provide authorized users with access through a single, secure cloud location, eliminating the need to bring external users behind the corporate firewall, issue laptops, or provide network IDs. -
Active Dossier
Maintain and visualize a holistic list of current and upcoming approved documents for a given product and
market. Automate based on submission management processes, and gain quick traceability to the relevant
submission details and document links in one place. -
Health Authority Interactions and Commitments
Retain and classify all correspondence with health authorities. Track questions from health authorities as
well as plan and author responses to questions. Also, track commitments to health authorities and related meetings.
Veeva RIM
Veeva Submissions is part of Veeva RIM, which streamlines global regulatory processes on a single, cloud-based platform. This enables life sciences companies to:
- Ensure teams are developing reliable regulatory content with high data integrity
- Coordinate regulatory efforts across headquarters, affiliates, and partners
- Respond faster to changing regulations
- Increase end-to-end process efficiency from submission planning to publishing