Features Brief
Veeva Batch Release Features Brief
In today’s pharmaceutical supply chain, the combination of applications and manual steps makes it challenging to speed up batch release without sacrificing due diligence and increasing risk.
Veeva Batch Release centralizes data and content from related Veeva solutions and third party systems, giving the batch disposition owner all the information needed to make a confident release decision.
For Veeva solutions such as QMS, QualityDocs, LIMS, and RIM, batch related content is automatically sourced and centralized. Veeva Batch Release also integrates with related solutions, such as inventory data from ERP, to give a complete picture of the batch.
Business Benefits
-
Increase efficiency.
Automate data aggregation, reviews, and traceability for faster, more confident release decisions.
-
Enable real-time visibility.
Review real-time batch status across data and content from QMS, LIMS, ERP, and regulatory solutions, and more.
-
Greater compliance.
Standardize and enforce the rules for quality checks, increasing confidence in decision making and reducing the risk of recall.
Features
-
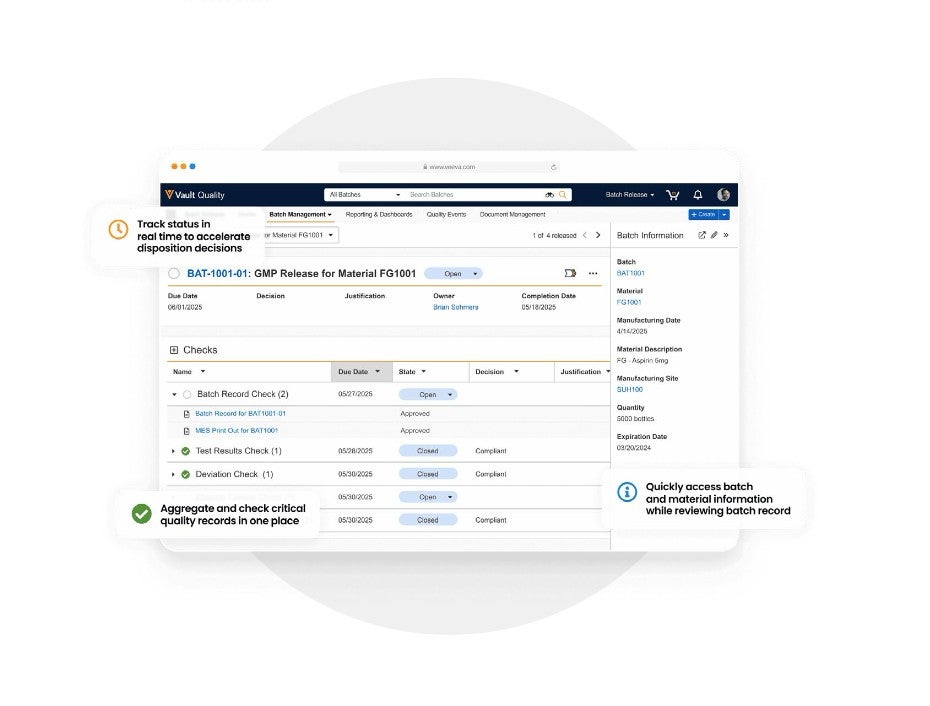
Release Dashboard
Monitor batches as they progress through key milestones and quality checks in a single, interactive view with clear visual indicators.
-
Configurable Workflows
Modify the best practice workflows to meet the needs of your supply chain.
-
Intuitive Interface
Drive productivity through a seamless, one-stop shop for batch planning, execution, and content.
-
Smart Document Management
Generate draft certificates and create placeholders for key documents seamlessly through Veeva QualityDocs integration.
-
Data and Content Aggregation
Assemble relevant information from manufacturing, lab, inventory, quality, and all other areas that define a batch’s story.
-
Reports and Metrics
Track critical indicators of efficiency, schedule adherence, and quality (right-first-time).
-
Automated Checks
Use system-driven evaluations and verifications to ensure the right content is in place and compliant.
-
System Integration
Access all relevant data and perform necessary tasks within a single application, eliminating data silos.
-
Comprehensive Genealogy
Track and view the ingredients used in each batch for end-to-end traceability.
-
Scheduling and Prioritization
Manage due dates for release decisions and their deliverables to keep teams aligned on priorities.
-
Release Requirement Management
Establish and enforce the rules for each compliance check and release decision to take risk out of the decision point.
About Veeva Quality Cloud
Veeva Quality Cloud accelerates the manufacturing of high-quality products to a greater number of patients. The cloud platform unifies applications, processes, and partners across content management, training, quality management systems (QMS), and QC lab solutions (LIMS).
Share insights, generate ideas with peers, and hear more tips in the Veeva Batch Release Community on Veeva Connect.