Features Brief
Veeva MedComms Features Brief
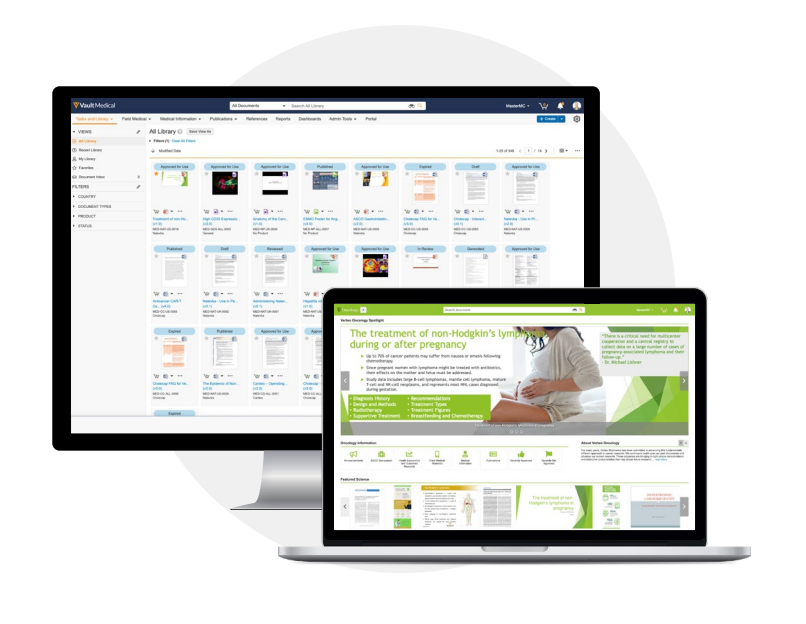
A scientific communications and content management solution tailored for global
medical affairs teams.
With increased complexity in all areas of healthcare, medical affairs teams are under greater pressure to provide consistent and accurate scientific information to an expanding number of stakeholders. Siloed systems, lack of version control, and disconnected business processes can impact the distribution of important content to the field and HCPs, degrading credibility.
Veeva MedComms helps centralize your scientific content in a single, global solution to rapidly deliver the information your stakeholders need, when they need it, in the format they prefer. A modern, cloud-based solution, Veeva MedComms speeds approvals, ensures content accuracy, and gives medical affairs more control over their content lifecycle.
Benefits
-
Digitize scientific communications platforms.
Manage scientific data and key messages centrally. Use scientific statements to create content with consistent language and referencing. Gain visibility into how and where key messages are being used externally to inform future activities. -
Accelerate medical content creation and approval.
Improve efficiency with one global solution for managing the end-to-end lifecycle of scientific content from creation to distribution. -
Ensure consistency across channels.
Quickly and easily distribute compliant content to field teams and medical information through native integration with Vault CRM and Veeva MedInquiry. -
Take ownership of the medical content lifecycle.
Establish a medical-specific content operating model and governance to drive more agile decision making. Equip teams with tailored dashboards and workflows to help them work more efficiently.
End-to-End Solution for Medical Communications
-
Plan
- Create and maintain Scientific Communications Platforms
- Access 1.5+ million prescriber email addresses from trusted industry sources
- Automatically link scientific statements and substantiating references to content
- Track statement usage to inform future activities
-
Collaborate
- Create and review content in a single, secure solution for all contributors
- Author collaboratively with Microsoft Office and comprehensive annotation
capabilities - Get a head start with template and reference libraries
-
Approve
- Establish flexible and easy review and approval processes for medical affairs or restrictions on
email data use - Track progress in real time and review full audit trail
- Ensure compliance with controlled access, expiration, and electronic signature capture
- Establish flexible and easy review and approval processes for medical affairs or restrictions on
-
Manage
- Search and filter with speed
- Maintain extensive file types and rich digital media
- View detailed dashboards to get a clear picture of progress and performance (content volume and usage, approval times, content usage)
-
Share
- Create local content derived from global assets while maintaining traceability back to original sources
- Tailor documents to meet local requirements and regulations with region-specific approval process
- Easily configure portals to showcase new content
-
Publish
- Disseminate content automatically through a variety of channels
- Integrate seamlessly with Vault CRM, Approved Email, Engage, and Veeva MedInquiry
- Share content to the web (via HCP self-service portals) through Vault digital publishing
Connected medical ecosystem built on Veeva Vault Platform
- Leverages the latest cloud technology, is IQ/OQ validated, PQ ready and easily delivered through the web.
- Supports access to the wider medical ecosystem with an open, published API to let customers easily integrate with other platforms and channels.
- Allows external system users to interact with content and data that resides in Veeva MedComms.