Features Brief
Veeva OpenData Clinical Features Brief
Streamline Trial Management with Clean Investigator and Site Data.
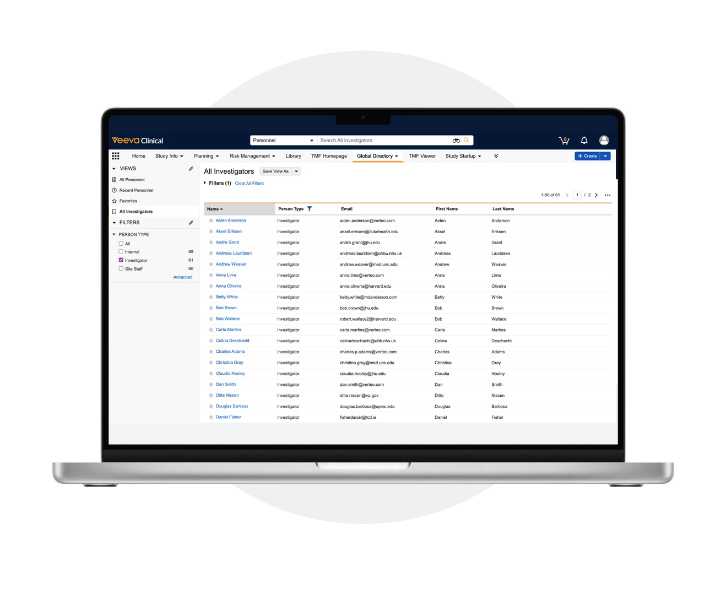
Veeva OpenData Clinical provides accurate investigator and research site reference data. It addresses common challenges such as incomplete information, costly manual updates, and inconsistent reporting. This is achieved through automated daily updates delivered directly within Veeva Clinical applications.
A common record ID creates a unified view of investigator interactions across Veeva Clinical, Medical, and Commercial systems, preventing duplicates and improving operations. Veeva data stewards curate the data with a quick Data Change Request (DCR) process, and it becomes available via Direct Data API for downstream system updates.
OpenData Clinical enhances data visibility, strengthens site relationships, and accelerates trial execution with continuously clean data and a comprehensive view of investigators.
Product Screenshot
Business Benefits
-
Ensure accurate reference data
Achieve a single source of truth by centralizing data ingestion and leveraging proven global collection and cleansing methods.
-
Eliminate manual data processes
Automate daily updates directly in Veeva Clinical applications, replacing costly manual processes and increasing time for site and patient activities.
-
Gain insight and enhance visibility
Gain a 360-degree view of an investigator across all clinical applications and support better reporting with continually reliable data.
Features:
-
Comprehensive data directory:
Access over 190,000 site and 230,000 investigator records with confirmed links to trial registries, spanning 70 countries and all therapeutic areas.
-
Common ID:
Ensure a unified view and understanding of investigator or site interactions, clean reporting across clinical operations, and seamless connections with other clinical trial systems.
-
Curated by Veeva:
Eliminate manual data cleanup by leveraging 350 Veeva data stewards who source, review, and update all records.
-
Automatic, daily updates:
Replace costly data update processes with automatic daily updates directly in Veeva Clinical applications.
-
Fast DCR process:
Expedite the data change request process for global directory updates and additions.
-
Integrate with Veeva CTMS and the Veeva Clinical Platform for accessible data via a standard OpenData user interface or Direct Data API.
-
Common data architecture:
Accelerate insights and increase efficiency with a common data architecture that integrates systems with connected entities and values.
-
Reporting and insights:
Enable enhanced reporting and insights with access to Vault reporting, data warehouses, and BI tools.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution. Streamlining processes and improving data visibility from start-up through closeout accelerates timelines and enhances collaboration across sponsors, sites, and CROs.